Difference between revisions of "Benzalkonium chloride"
(username removed) |
|||
| (3 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | An odorless antiseptic compound widely used as a disinfectant in detergents. Benzalkonium chloride is active at low concentrations and has been used to kill bacteria, [ | + | An odorless antiseptic compound widely used as a disinfectant in detergents. Benzalkonium chloride is active at low concentrations and has been used to kill bacteria, [[fungus|fungi]], [[algae]], and [[lichen|lichens]]. It does not kill [[spore|spores]]. This type of quaternary ammonium compound has reduced effectiveness in solutions containing hard water, salts, or organic compounds. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 9: | Line 9: | ||
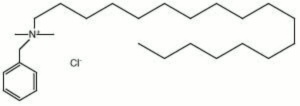
[[[SliderGallery rightalign|benzalkonium chloride.jpg~Chemical structure]]] | [[[SliderGallery rightalign|benzalkonium chloride.jpg~Chemical structure]]] | ||
| − | == | + | == Risks == |
| + | |||
| + | * Corrosive. | ||
| + | * Hygroscopic. | ||
| + | * Contact causes skin irritation. | ||
| + | * Toxic by ingestion and inhalation. LD50 = 240 mg/kg. | ||
| + | * An overdose may cause shortness of breath, cyanosis, CNS depression, low blood pressure, coma. | ||
| + | * Found to be a teratogen in animals. | ||
| + | * Fisher Scientific: [https://fscimage.fishersci.com/msds/87924.htm MSDS] | ||
| + | |||
| + | ==Physical and Chemical Properties== | ||
Soluble in sorbitol solutions, glycerol, ether, water | Soluble in sorbitol solutions, glycerol, ether, water | ||
| Line 19: | Line 29: | ||
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 0.98 | + | | 0.98 g/ml |
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* ''The Merck Index'', Susan Budavari (ed.), Merck Research Labs, Whitehouse Station, NJ, 12th Edition, 1996 | * ''The Merck Index'', Susan Budavari (ed.), Merck Research Labs, Whitehouse Station, NJ, 12th Edition, 1996 | ||
Latest revision as of 17:26, 2 May 2022
Description
An odorless antiseptic compound widely used as a disinfectant in detergents. Benzalkonium chloride is active at low concentrations and has been used to kill bacteria, fungi, Algae, and lichens. It does not kill spores. This type of quaternary ammonium compound has reduced effectiveness in solutions containing hard water, salts, or organic compounds.
Synonyms and Related Terms
alkyldimethylbenzyl ammonium chloride; alkyl dimethylbenzyl ammonium chloride; alkyldimethyl(phenylmethyl)quaternary ammonium chlorides; quaternary ammonium compounds; alkylbenzyldimethyl chloride; Zephiran chloride; Preventol R50, R80, R90 [Bayer]; Hyamine 3500 [Rohm & Haas]; Céquartyl [Rhone Poulenc]; Neo-desogen [Ciba Geigy]; Benirol; BTC; Zephirol
Risks
- Corrosive.
- Hygroscopic.
- Contact causes skin irritation.
- Toxic by ingestion and inhalation. LD50 = 240 mg/kg.
- An overdose may cause shortness of breath, cyanosis, CNS depression, low blood pressure, coma.
- Found to be a teratogen in animals.
- Fisher Scientific: MSDS
Physical and Chemical Properties
Soluble in sorbitol solutions, glycerol, ether, water
| CAS | 8001-54-5 |
|---|---|
| Density | 0.98 g/ml |
Resources and Citations
- The Merck Index, Susan Budavari (ed.), Merck Research Labs, Whitehouse Station, NJ, 12th Edition, 1996
- MSDS Sheet Comment: Fisher Scientific 8/20/2002
- G.Caneva, M.P.Nugari, O.Salvadori, Biology in the Conservation of Works of Art, ICCROM, Rome, 1991