Difference between revisions of "Butyl acetate"
Jump to navigation
Jump to search
(username removed) |
|||
| (3 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
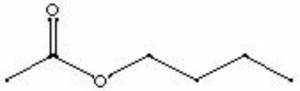
| − | + | [[[SliderGallery rightalign|butyl acetate.jpg~Chemical structure]]] | |
| − | A colorless, sweet-smelling liquid. Butyl acetate has been used as a solvent for [ | + | A colorless, sweet-smelling liquid. Butyl acetate has been used as a solvent for [[cellulose nitrate]] products such as [[celluloid]], [[pyroxylin]] lacquers, and airplane wing [[dope]]. It is also used in the manufacture of photographic film, artificial leather, and [[safety glass]]. Because butyl acetate is not [[hygroscopic]] and evaporates slowly, it produces smooth shiny, transparent films. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 7: | Line 7: | ||
n-butyl acetate; acetic acid butyl ester | n-butyl acetate; acetic acid butyl ester | ||
| − | |||
| − | == | + | == Risks == |
| + | |||
| + | * Narcotic in high concentrations. | ||
| + | * Skin contact may cause irritation. | ||
| + | * Flammable. | ||
| + | * ThermoFisher: [https://www.fishersci.com/store/msds?partNumber=AC327850010&productDescription=BUTYL+ACETATE%2C+ELECTRONI+1LT&vendorId=VN00032119&countryCode=US&language=en SDS] | ||
| + | |||
| + | ==Physical and Chemical Properties== | ||
Miscible with ethanol, ether and most hydrocarbon solvents. | Miscible with ethanol, ether and most hydrocarbon solvents. | ||
| Line 22: | Line 28: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | -75 | + | | -75 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 0.8826 | + | | 0.8826 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 34: | Line 40: | ||
|- | |- | ||
! scope="row"| Boiling Point | ! scope="row"| Boiling Point | ||
| − | | 125-126 | + | | 125-126 C |
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | * | + | * R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 |
| − | * | + | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 |
| − | * | + | * Michael McCann, ''Artist Beware'', Watson-Guptill Publications, New York City, 1979 |
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 1597 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 1597 | ||
Latest revision as of 13:38, 11 May 2022
Description
A colorless, sweet-smelling liquid. Butyl acetate has been used as a solvent for Cellulose nitrate products such as Celluloid, Pyroxylin lacquers, and airplane wing Dope. It is also used in the manufacture of photographic film, artificial leather, and Safety glass. Because butyl acetate is not Hygroscopic and evaporates slowly, it produces smooth shiny, transparent films.
Synonyms and Related Terms
n-butyl acetate; acetic acid butyl ester
Risks
- Narcotic in high concentrations.
- Skin contact may cause irritation.
- Flammable.
- ThermoFisher: SDS
Physical and Chemical Properties
Miscible with ethanol, ether and most hydrocarbon solvents.
| Composition | CH3COOCH2CH2CH2CH3 |
|---|---|
| CAS | 123-86-4 |
| Melting Point | -75 C |
| Density | 0.8826 g/ml |
| Molecular Weight | mol. wt. = 116.2 |
| Refractive Index | 1.2951 |
| Boiling Point | 125-126 C |
Resources and Citations
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 1597