Difference between revisions of "Cobaltous chloride"
Jump to navigation
Jump to search
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
|||
| Line 3: | Line 3: | ||
Pale blue, flaky crystals. Cobaltous chloride is most commonly used as a color indicator of moisture. On exposure to moist air, anhydrous cobaltous chloride absorbs water forming pink-color hexahydrate crystals. The blue anhydrous form can be regenerated by heating at 52-56 C. Cobaltous chloride is added to silica gel and activated alumina type desiccant as a moisture indicator. It has also been used in indicator paper. | Pale blue, flaky crystals. Cobaltous chloride is most commonly used as a color indicator of moisture. On exposure to moist air, anhydrous cobaltous chloride absorbs water forming pink-color hexahydrate crystals. The blue anhydrous form can be regenerated by heating at 52-56 C. Cobaltous chloride is added to silica gel and activated alumina type desiccant as a moisture indicator. It has also been used in indicator paper. | ||
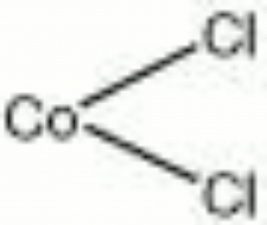
| − | + | [[[SliderGallery rightalign|cobaltous chloride.jpg~Chemical structure]]] | |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
cobalt chloride; cobalt dichloride; cobalt (II) chloride; Cobalt(II)-chlorid (Deut.) | cobalt chloride; cobalt dichloride; cobalt (II) chloride; Cobalt(II)-chlorid (Deut.) | ||
| − | [ | + | == Risks == |
| + | |||
| + | * Skin contact may cause allergies, especially on elbows, neck and ankles. | ||
| + | * Chronic inhalation may cause asthma. | ||
| + | * Ingestion may cause vomiting, diarrhea and the sensation of hotness. | ||
| + | * Fisher Scientific: [https://fscimage.fishersci.com/msds/95188.htm#:~:text=MSDS%20Name%3ACobaltous%20Chloride%201%20M%20Solution,Catalog%20Numbers%3AS79972%20Synonyms%3ACobalt%20%28II%29%20chloride%3B%20Cobalt%20muriate MSDS] | ||
| − | == | + | ==Physical and Chemical Properties== |
Soluble in water, alcohols, acetone, ether, glycerol, pyridine. | Soluble in water, alcohols, acetone, ether, glycerol, pyridine. | ||
| Line 23: | Line 28: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 735 | + | | 735 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 3.348 | + | | 3.348 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 32: | Line 37: | ||
|- | |- | ||
! scope="row"| Boiling Point | ! scope="row"| Boiling Point | ||
| − | | 1049 | + | | 1049 C |
|} | |} | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
== Additional Images == | == Additional Images == | ||
| Line 47: | Line 46: | ||
</gallery> | </gallery> | ||
| − | + | ==Resources and Citations== | |
| − | == | ||
* G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 406 | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 406 | ||
Revision as of 13:28, 30 May 2022
Description
Pale blue, flaky crystals. Cobaltous chloride is most commonly used as a color indicator of moisture. On exposure to moist air, anhydrous cobaltous chloride absorbs water forming pink-color hexahydrate crystals. The blue anhydrous form can be regenerated by heating at 52-56 C. Cobaltous chloride is added to silica gel and activated alumina type desiccant as a moisture indicator. It has also been used in indicator paper.
Synonyms and Related Terms
cobalt chloride; cobalt dichloride; cobalt (II) chloride; Cobalt(II)-chlorid (Deut.)
Risks
- Skin contact may cause allergies, especially on elbows, neck and ankles.
- Chronic inhalation may cause asthma.
- Ingestion may cause vomiting, diarrhea and the sensation of hotness.
- Fisher Scientific: MSDS
Physical and Chemical Properties
Soluble in water, alcohols, acetone, ether, glycerol, pyridine.
| Composition | CoCl2 |
|---|---|
| CAS | 7646-79-9 |
| Melting Point | 735 C |
| Density | 3.348 g/ml |
| Molecular Weight | mol. wt. = 129.84 |
| Boiling Point | 1049 C |
Additional Images
Resources and Citations
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 406
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Dictionary of Paper, American Paper Institute, New York, Fourth Edition, 1980
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 2498
- Wikipedia, the free encyclopedia, at http://www.wikipedia.com Comment: http://en.wikipedia.org/wiki/Cobalt_chloride (Accessed Jan. 15, 2006)
- B. Gascoigne, How to Identify Prints, Thames & Hudson, London, 2004
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: ref. index=