Difference between revisions of "Smalt"
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
|||
| (5 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
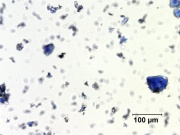
| − | [[File:41_Smalt_200X.jpg|thumb|Smalt]] | + | [[File:41_Smalt_200X.jpg|thumb|Smalt at 200x visible light]] |
== Description == | == Description == | ||
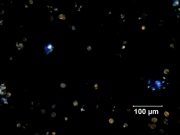
| − | + | [[File:41_Smalt_200X_pol.jpg|thumb|Smalt at 200x polarized light]] | |
A coarsely ground blue potassium glass containing small amounts of [[cobalt oxide]], which provides the source of the blue color. Smalt was used as a paint pigment in 11th - 13th century Chinese wall painting. It was used in European paintings from the early 15th-century, probably first in Germany and subsequently in Italy, and became widespread in the 16th- and particularly, the 17th-centuries. Be the 17th-century, the Netherlands had become a manufacturing center of large output, using cobalt ores imported from Germany. The pigment was exported from there all over Europe. Smalt remained a pigment available to painters until the 19th-century, but it had been somewhat displaced by [[ Prussian blue]] from the early 18th-century onwards. The composition of smalt varies with manufacture. It is prepared by fusing cobalt oxide with [[silica]] and [[potassium carbonate]] (derived from wood ash). In addition to its characteristic elements of silicon, potassium, and cobalt, smaller quantities of arsenic are often also detected, and this arises from the original cobalt ore source. Smalt can be a stable, lightfast pigment, but it has poor covering power. In oil media, smalt frequently discolors to a gray or grayish brown tone through a complex interaction of pigment and medium, which involves leaching of potassium and cobalt from the particles and the formation of potassium soaps in the paint film. The process is impeded by admixture with lead white, but worsened by moisture. Smalt has been used as a blue colorant in paints, glazes, glass, bluing paper, laundry blue, starch, textile, and rubber. | A coarsely ground blue potassium glass containing small amounts of [[cobalt oxide]], which provides the source of the blue color. Smalt was used as a paint pigment in 11th - 13th century Chinese wall painting. It was used in European paintings from the early 15th-century, probably first in Germany and subsequently in Italy, and became widespread in the 16th- and particularly, the 17th-centuries. Be the 17th-century, the Netherlands had become a manufacturing center of large output, using cobalt ores imported from Germany. The pigment was exported from there all over Europe. Smalt remained a pigment available to painters until the 19th-century, but it had been somewhat displaced by [[ Prussian blue]] from the early 18th-century onwards. The composition of smalt varies with manufacture. It is prepared by fusing cobalt oxide with [[silica]] and [[potassium carbonate]] (derived from wood ash). In addition to its characteristic elements of silicon, potassium, and cobalt, smaller quantities of arsenic are often also detected, and this arises from the original cobalt ore source. Smalt can be a stable, lightfast pigment, but it has poor covering power. In oil media, smalt frequently discolors to a gray or grayish brown tone through a complex interaction of pigment and medium, which involves leaching of potassium and cobalt from the particles and the formation of potassium soaps in the paint film. The process is impeded by admixture with lead white, but worsened by moisture. Smalt has been used as a blue colorant in paints, glazes, glass, bluing paper, laundry blue, starch, textile, and rubber. | ||
| − | |||
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
bleu de smalt (Fr.); smalt (Fr., Ned.); Streublau (Deut.) Smalte (Deut.); Zafferblau (Deut.); azzurro di smalto (It.); blu di smalto (It.); smaltino (It.); esmalte (Esp., Port.); hana konjo (Jap.); smalta (Pol.); smalto (Gr.); azure blue; Dumont blue; Hungary blue; Saxon blue; eschel; zaffre; zaffera; royal blue; blue glass; starch blue; king's blue | bleu de smalt (Fr.); smalt (Fr., Ned.); Streublau (Deut.) Smalte (Deut.); Zafferblau (Deut.); azzurro di smalto (It.); blu di smalto (It.); smaltino (It.); esmalte (Esp., Port.); hana konjo (Jap.); smalta (Pol.); smalto (Gr.); azure blue; Dumont blue; Hungary blue; Saxon blue; eschel; zaffre; zaffera; royal blue; blue glass; starch blue; king's blue | ||
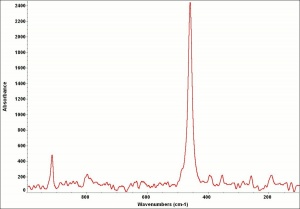
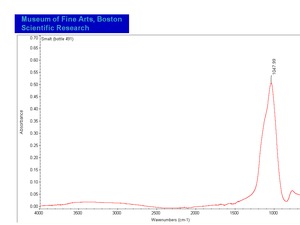
| − | [[[SliderGallery rightalign|SmaltUCL.jpg~Raman| | + | [[[SliderGallery rightalign|SmaltUCL.jpg~Raman|Smalt (bottle 491).TIF~FTIR (MFA)]]] |
| − | == | + | == Risks == |
| − | + | * No significant hazards | |
| + | ==Physical and Chemical Properties== | ||
| − | Conchoidal fracture, with sharp edges and splinter. No birefringence, no pleochroism. Pale blue to deep purple particles are transparent. | + | * Unaffected by most acids or alkalis. |
| − | + | * Degraded samples may be soluble in dilute acids. | |
| − | + | * Conchoidal fracture, with sharp edges and splinter. | |
| − | + | * No birefringence, no pleochroism. | |
| − | + | * Pale blue to deep purple particles are transparent. | |
| − | + | * Composition = K, Al, Co silicate | |
| − | + | * Refractive Index = 1.46-1.55 | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
== Comparisons == | == Comparisons == | ||
| − | [[media: | + | [[media:download_file_499.pdf|Characteristics of Common Blue Pigments]] |
| − | |||
| − | |||
| − | == | + | ==Resources and Citations== |
| + | * B. Muhlethaler and J. Thissen, "Smalt", ''Artists Pigments'', Vol. 2., A. Roy ed., Oxford University Press, Oxford, 1993. | ||
* Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, ''Pigment Compendium'', Elsevier Butterworth-Heinemann, Oxford, 2004 | * Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, ''Pigment Compendium'', Elsevier Butterworth-Heinemann, Oxford, 2004 | ||
| − | * | + | * Ashok Roy, Contributed information, November 2007 |
* Ralph Mayer, ''A Dictionary of Art Terms and Techniques'', Harper and Row Publishers, New York, 1969 (also 1945 printing) | * Ralph Mayer, ''A Dictionary of Art Terms and Techniques'', Harper and Row Publishers, New York, 1969 (also 1945 printing) | ||
| Line 72: | Line 60: | ||
* Book and Paper Group, ''Paper Conservation Catalog'', AIC, 1984, 1989 | * Book and Paper Group, ''Paper Conservation Catalog'', AIC, 1984, 1989 | ||
| − | * Art and Architecture Thesaurus Online, | + | * Art and Architecture Thesaurus Online, https://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 |
| − | |||
| − | |||
| + | * Pigments Through The Ages - http://webexhibits.org/pigments/indiv/overview/smalt.html - Refractive index: 1.46 - 1.55 | ||
| + | Record content reviewed by EU-Artech, November 2007. | ||
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 12:40, 31 May 2022
Description
A coarsely ground blue potassium glass containing small amounts of Cobalt oxide, which provides the source of the blue color. Smalt was used as a paint pigment in 11th - 13th century Chinese wall painting. It was used in European paintings from the early 15th-century, probably first in Germany and subsequently in Italy, and became widespread in the 16th- and particularly, the 17th-centuries. Be the 17th-century, the Netherlands had become a manufacturing center of large output, using cobalt ores imported from Germany. The pigment was exported from there all over Europe. Smalt remained a pigment available to painters until the 19th-century, but it had been somewhat displaced by Prussian blue from the early 18th-century onwards. The composition of smalt varies with manufacture. It is prepared by fusing cobalt oxide with Silica and Potassium carbonate (derived from wood ash). In addition to its characteristic elements of silicon, potassium, and cobalt, smaller quantities of arsenic are often also detected, and this arises from the original cobalt ore source. Smalt can be a stable, lightfast pigment, but it has poor covering power. In oil media, smalt frequently discolors to a gray or grayish brown tone through a complex interaction of pigment and medium, which involves leaching of potassium and cobalt from the particles and the formation of potassium soaps in the paint film. The process is impeded by admixture with lead white, but worsened by moisture. Smalt has been used as a blue colorant in paints, glazes, glass, bluing paper, laundry blue, starch, textile, and rubber.
Synonyms and Related Terms
bleu de smalt (Fr.); smalt (Fr., Ned.); Streublau (Deut.) Smalte (Deut.); Zafferblau (Deut.); azzurro di smalto (It.); blu di smalto (It.); smaltino (It.); esmalte (Esp., Port.); hana konjo (Jap.); smalta (Pol.); smalto (Gr.); azure blue; Dumont blue; Hungary blue; Saxon blue; eschel; zaffre; zaffera; royal blue; blue glass; starch blue; king's blue
Risks
- No significant hazards
Physical and Chemical Properties
- Unaffected by most acids or alkalis.
- Degraded samples may be soluble in dilute acids.
- Conchoidal fracture, with sharp edges and splinter.
- No birefringence, no pleochroism.
- Pale blue to deep purple particles are transparent.
- Composition = K, Al, Co silicate
- Refractive Index = 1.46-1.55
Comparisons
Characteristics of Common Blue Pigments
Resources and Citations
- B. Muhlethaler and J. Thissen, "Smalt", Artists Pigments, Vol. 2., A. Roy ed., Oxford University Press, Oxford, 1993.
- Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, Pigment Compendium, Elsevier Butterworth-Heinemann, Oxford, 2004
- Ashok Roy, Contributed information, November 2007
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- The Dictionary of Art, Grove's Dictionaries Inc., New York, 1996 Comment: "Pigments"
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966 Comment: ref index=1.49-1.52
- M. Doerner, The Materials of the Artist, Harcourt, Brace & Co., 1934
- R.D. Harley, Artists' Pigments c. 1600-1835, Butterworth Scientific, London, 1982
- Artists' Pigments: A Handbook of their History and Characteristics, Ashok Roy (ed.), National Gallery of Art, Washington DC, Vol. 2, 1993 Comment: B. Muhlethaler and J. Thissen, "Smalt"
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 207
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982
- Dictionary of Building Preservation, Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996
- Thomas B. Brill, Light Its Interaction with Art and Antiquities, Plenum Press, New York City, 1980 Comment: ref. index = 1.45-1.52
- Book and Paper Group, Paper Conservation Catalog, AIC, 1984, 1989
- Art and Architecture Thesaurus Online, https://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000
- Pigments Through The Ages - http://webexhibits.org/pigments/indiv/overview/smalt.html - Refractive index: 1.46 - 1.55
Record content reviewed by EU-Artech, November 2007.