Difference between revisions of "Sodium oxalate"
Jump to navigation
Jump to search
(username removed) |
|||
| (One intermediate revision by one other user not shown) | |||
| Line 9: | Line 9: | ||
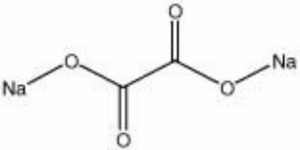
[[[SliderGallery rightalign|sodium oxalate.jpg~Chemical structure]]] | [[[SliderGallery rightalign|sodium oxalate.jpg~Chemical structure]]] | ||
| − | == | + | == Risks == |
| + | |||
| + | * Toxic by ingestion. | ||
| + | * Contact causes irritation. | ||
| + | * Fisher Scientific: [https://fscimage.fishersci.com/msds/96509.htm MSDS] | ||
| + | |||
| + | == Physical and Chemical Properties == | ||
Soluble in water. Insoluble in ethanol. | Soluble in water. Insoluble in ethanol. | ||
| Line 22: | Line 28: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 250-270 | + | | 250-270 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 2.34 | + | | 2.34 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 31: | Line 37: | ||
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 736 | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 736 | ||
Latest revision as of 08:58, 2 June 2022
Description
White, odorless hygroscopic solid. Sodium oxalate is used as a source of oxalate. It is used for finishing textiles, tanning leather, and blue printing.
Synonyms and Related Terms
ethandioic acid disodium salt
Risks
- Toxic by ingestion.
- Contact causes irritation.
- Fisher Scientific: MSDS
Physical and Chemical Properties
Soluble in water. Insoluble in ethanol.
| Composition | Na2C2O4 |
|---|---|
| CAS | 62-76-0 |
| Melting Point | 250-270 C |
| Density | 2.34 g/ml |
| Molecular Weight | mol. wt. = 134.0 |
Resources and Citations
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 736
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 8795