Difference between revisions of "Sodium sulfate, decahydrate"
Jump to navigation
Jump to search
(username removed) |
|||
| (2 intermediate revisions by one other user not shown) | |||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | Colorless, crystalline solid. Hydrated sodium sulfate occurs in nature as the mineral mirabilite. In the 17th century it was called Glauber's salt after a German chemist, Johann Glauber who extracted it from Hungarian spring water. Sodium sulfate is used in the manufacture of [ | + | Colorless, crystalline solid. Hydrated sodium sulfate occurs in nature as the mineral mirabilite. In the 17th century it was called Glauber's salt after a German chemist, Johann Glauber who extracted it from Hungarian spring water. Sodium sulfate is used in the manufacture of [[kraft%20paper|kraft paper]], [[paperboard|paperboard]], [[glass|glass]], [[ultramarine%20blue%2C%20synthetic|synthetic ultramarine blue]], and ceramic [[glaze|glaze]]. It is also used as a leveling agent in dyeing textiles to ensure even color acceptance. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 9: | Line 9: | ||
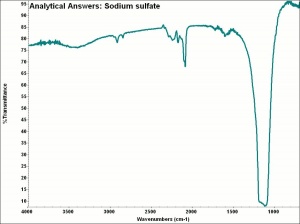
[[[SliderGallery rightalign|aaiNASO4.jpg~FTIR]]] | [[[SliderGallery rightalign|aaiNASO4.jpg~FTIR]]] | ||
| − | == | + | == Hazards and Safety == |
| + | |||
| + | * Nonflammable. | ||
| + | * ThermoFisher Scientific: [https://www.fishersci.com/store/msds?partNumber=AC611615000&productDescription=SODIUM+SULFATE+DECAHYDRAT+500G&vendorId=VN00033901&countryCode=US&language=en SDS] | ||
| + | |||
| + | == Physical and Chemical Properties == | ||
Soluble in water, glycerol. Insoluble in ethanol. | Soluble in water, glycerol. Insoluble in ethanol. | ||
| Line 22: | Line 27: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 33 | + | | 33 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 1.464 | + | | 1.464 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 31: | Line 36: | ||
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | * Grossi, "Acoustic emission monitoring to study sodium sulfate crystallization in monumental porous carbonate stone" ''Studies in Conservation'' 42 (1997), p. 115-125. | |
* G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 785 | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 785 | ||
Latest revision as of 11:25, 2 June 2022
Description
Colorless, crystalline solid. Hydrated sodium sulfate occurs in nature as the mineral mirabilite. In the 17th century it was called Glauber's salt after a German chemist, Johann Glauber who extracted it from Hungarian spring water. Sodium sulfate is used in the manufacture of Kraft paper, Paperboard, Glass, synthetic ultramarine blue, and ceramic Glaze. It is also used as a leveling agent in dyeing textiles to ensure even color acceptance.
Synonyms and Related Terms
sodium sulfate, hydrated; sodium sulfate crystals; mirabilite; Glauber's salt; sal mirabile; crazy water crystals
Hazards and Safety
- Nonflammable.
- ThermoFisher Scientific: SDS
Physical and Chemical Properties
Soluble in water, glycerol. Insoluble in ethanol.
| Composition | Na2SO4 - 10H2O |
|---|---|
| CAS | 7727-73-3 |
| Melting Point | 33 C |
| Density | 1.464 g/ml |
| Molecular Weight | mol. wt. = 322.17 |
Resources and Citations
- Grossi, "Acoustic emission monitoring to study sodium sulfate crystallization in monumental porous carbonate stone" Studies in Conservation 42 (1997), p. 115-125.
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 785
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Dictionary of Paper, American Paper Institute, New York, Fourth Edition, 1980
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 8829
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- John and Margaret Cannon, Dye Plants and Dyeing, Herbert Press, London, 1994
- Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985