Difference between revisions of "Sucrose"
Jump to navigation
Jump to search
m (Text replace - "\[http:\/\/cameo\.mfa\.org\/materials\/fullrecord\.asp\?name=([^\s]+)\s(.*)\]" to "$2") |
|||
| Line 9: | Line 9: | ||
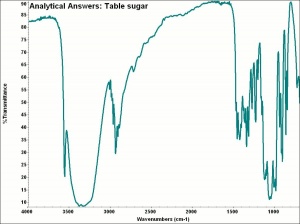
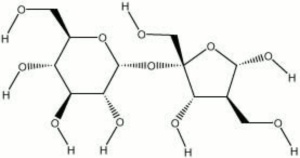
[[[SliderGallery rightalign|aaiSUGAR.jpg~FTIR|sucrose.jpg~Chemical structure]]] | [[[SliderGallery rightalign|aaiSUGAR.jpg~FTIR|sucrose.jpg~Chemical structure]]] | ||
| − | == | + | == Risks == |
| − | + | * Combustible. | |
| + | * High concentrations may cause irritation. | ||
| + | * ThermoFisher: [https://www.fishersci.com/store/msds?partNumber=BP2201&productDescription=SUCROSE+1KG&vendorId=VN00033897&countryCode=US&language=en SDS] | ||
| − | Chars with the smell of caramel. | + | == Physical and Chemical Properties == |
| + | |||
| + | * Soluble in water. Slightly soluble in ethanol, methanol, glycerol, pyridine. | ||
| + | * Chars with the smell of caramel. | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 24: | Line 29: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 160 (dec) | + | | 160 C (dec) |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 1.5877 | + | | 1.5877 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 33: | Line 38: | ||
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | * A Morgos, S.Imazu "A Conservation Method for Waterlogged Wood using a Sucrose-Mannitol Mixture" ICOM Preprints, Washington DC, 1993, p.266-272. | |
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
Latest revision as of 12:34, 6 June 2022
Description
Hard, white, crystals with a sweet taste. Sucrose occurs naturally in sugar cane, sugar beets, Sugar maple, and sorghum. It is extracted with water and purified with Lime or Carbon. Sucrose is a disaccharide that hydrolyzes to Glucose and Fructose. It is primarily used as a sweetening agent in foods and drinks. It has also been used in the manufacture of Polyurethane foams, printing inks, and transparent soaps. Sucrose has been used in conjunction with Mannitol for the impregnation of waterlogged Wood (Morgos and Imazu, 1993).
Synonyms and Related Terms
table sugar; saccharose; cane sugar; beet sugar
Risks
- Combustible.
- High concentrations may cause irritation.
- ThermoFisher: SDS
Physical and Chemical Properties
- Soluble in water. Slightly soluble in ethanol, methanol, glycerol, pyridine.
- Chars with the smell of caramel.
| Composition | C12H22O11 |
|---|---|
| CAS | 57-50-1 |
| Melting Point | 160 C (dec) |
| Density | 1.5877 g/ml |
| Molecular Weight | mol. wt. = 342.3 |
Resources and Citations
- A Morgos, S.Imazu "A Conservation Method for Waterlogged Wood using a Sucrose-Mannitol Mixture" ICOM Preprints, Washington DC, 1993, p.266-272.
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- John S. Mills, Raymond White, The Organic Chemistry of Museum Objects, Butterworth Heineman, London, 2nd ed., 1994
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 9051