Difference between revisions of "Sugar"
Jump to navigation
Jump to search
| (2 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
| − | [[File:2006.217-SC209051.jpg|thumb| | + | [[File:sugarcane press MFA.jpg|thumb|Sugarcane grinder<br>MFA# 49.239]][[File:2006.217-SC209051.jpg|thumb|Sugar and Salt<br>MFA Acc. #: 2006.217]] |
== Description == | == Description == | ||
A photosynthesis product in plants. Sugar is an important source of metabolic energy in foods and its formation in plants is an essential factor in the life process. A sugar is a small [[carbohydrate|carbohydrate]] composed of one, two, or more saccharose groups. The chief monosaccharides are [[glucose|glucose]] (dextrose) and [[fructose|fructose]] (levulose). Some primary disaccharides are [[sucrose|sucrose]] (from cane or beet sugar); [[lactose|lactose]] (from milk); [[maltose|maltose]] (from [[starch|starch]]); and cellobiose (from [[cellulose|cellulose]]). Table sugar is primarily composed of sucrose. | A photosynthesis product in plants. Sugar is an important source of metabolic energy in foods and its formation in plants is an essential factor in the life process. A sugar is a small [[carbohydrate|carbohydrate]] composed of one, two, or more saccharose groups. The chief monosaccharides are [[glucose|glucose]] (dextrose) and [[fructose|fructose]] (levulose). Some primary disaccharides are [[sucrose|sucrose]] (from cane or beet sugar); [[lactose|lactose]] (from milk); [[maltose|maltose]] (from [[starch|starch]]); and cellobiose (from [[cellulose|cellulose]]). Table sugar is primarily composed of sucrose. | ||
| − | |||
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
sucre (Fr.); sucrose; cane sugar; beet sugar | sucre (Fr.); sucrose; cane sugar; beet sugar | ||
| − | |||
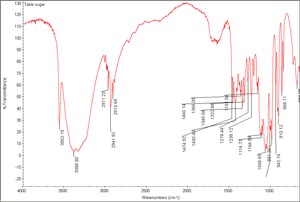
[[[SliderGallery rightalign|Table sugar.TIF~FTIR (MFA)]]] | [[[SliderGallery rightalign|Table sugar.TIF~FTIR (MFA)]]] | ||
| − | == | + | == Physical and Chemical Properties == |
| − | Soluble in water. Hygroscopic. | + | * Soluble in water. |
| − | + | * Hygroscopic. | |
| − | + | * Density = 1.59 g/ml | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
== Additional Images == | == Additional Images == | ||
| − | |||
<gallery> | <gallery> | ||
| + | File:image5_canesugarreal.jpg|Stalks of sugarcane | ||
File:image4_canesugarcommercial.jpg|Cane Sugar | File:image4_canesugarcommercial.jpg|Cane Sugar | ||
</gallery> | </gallery> | ||
| + | == Resources and Citations == | ||
| − | + | * V.Daniels, G.Lohneis, "Deterioration of Sugar Artifacts" ''Studies in Conservation'' 42:17-26, 1997. | |
* G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 777 | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 777 | ||
Latest revision as of 12:44, 6 June 2022
Description
A photosynthesis product in plants. Sugar is an important source of metabolic energy in foods and its formation in plants is an essential factor in the life process. A sugar is a small Carbohydrate composed of one, two, or more saccharose groups. The chief monosaccharides are Glucose (dextrose) and Fructose (levulose). Some primary disaccharides are Sucrose (from cane or beet sugar); Lactose (from milk); Maltose (from Starch); and cellobiose (from Cellulose). Table sugar is primarily composed of sucrose.
Synonyms and Related Terms
sucre (Fr.); sucrose; cane sugar; beet sugar
Physical and Chemical Properties
- Soluble in water.
- Hygroscopic.
- Density = 1.59 g/ml
Additional Images
Resources and Citations
- V.Daniels, G.Lohneis, "Deterioration of Sugar Artifacts" Studies in Conservation 42:17-26, 1997.
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 777
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980