Difference between revisions of "Tungstic acid"
Jump to navigation
Jump to search
(username removed) |
|||
| (2 intermediate revisions by one other user not shown) | |||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | Yellow powder. Tungstic acid is used as a [ | + | Yellow powder. Tungstic acid is used as a [[mordant|mordant]] for dyeing [[textile|textiles]]. It is also used as a [[colorant|colorant]] in [[plastic|plastics]]. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 9: | Line 9: | ||
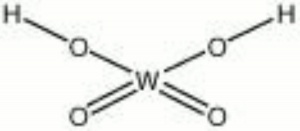
[[[SliderGallery rightalign|tungstic acid.jpg~Chemical structure]]] | [[[SliderGallery rightalign|tungstic acid.jpg~Chemical structure]]] | ||
| − | == | + | == Risks == |
| + | |||
| + | * Toxic by ingestion and inhalation. | ||
| + | * Fisher Scientific: [https://fscimage.fishersci.com/msds/33757.htm MSDS] | ||
| + | |||
| + | ==Physical and Chemical Properties== | ||
Soluble in hydrofluoric acid and alkalis. Insoluble in water. | Soluble in hydrofluoric acid and alkalis. Insoluble in water. | ||
| Line 22: | Line 27: | ||
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 5.5 | + | | 5.5 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 28: | Line 33: | ||
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 694 | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 694 | ||
Latest revision as of 08:54, 22 June 2022
Description
Yellow powder. Tungstic acid is used as a Mordant for dyeing textiles. It is also used as a Colorant in plastics.
Synonyms and Related Terms
wolframic acid; orthotungstic acid
Risks
- Toxic by ingestion and inhalation.
- Fisher Scientific: MSDS
Physical and Chemical Properties
Soluble in hydrofluoric acid and alkalis. Insoluble in water.
| Composition | H2WO4 |
|---|---|
| CAS | 7783-03-1 |
| Density | 5.5 g/ml |
| Molecular Weight | mol. wt. = 249.88 |
Resources and Citations
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 694
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 9948
- Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985