Difference between revisions of "Vulcanite"
| (One intermediate revision by the same user not shown) | |||
| Line 1: | Line 1: | ||
| − | [[File:02.222-E11509CR-d1.jpg|thumb|]] | + | [[File:02.222-E11509CR-d1.jpg|thumb|Devotion panel<br>MFA# 02.222]] |
== Description == | == Description == | ||
An old brand name for a hard, dark, shiny rubber developed in the 1830s. The term Vulcanite was used for marketing in the U.S. while the name [[Ebonite|Ebonite]] was used in England. Vulcanite is produced by heating natural rubber with 10-32% sulfur. This is a much higher sulfur concentration than is usually used in vulcanization and the process results in an extremely brittle, dense moldable product. Vulcanite is naturally dark brown and opaque, but on exposure to light and moisture, the sulfur on the surface can oxidize forming a yellowish to greenish tinge. This surface is also acidic and can corrode adjacent metals. When warmed, vulcanite exudes a sulfurous odor. It was used to make combs, buttons, brooches, boxes, medallions, pens, and pencils. Vulcanite had good electrical insulation properties and was widely used for insulation. | An old brand name for a hard, dark, shiny rubber developed in the 1830s. The term Vulcanite was used for marketing in the U.S. while the name [[Ebonite|Ebonite]] was used in England. Vulcanite is produced by heating natural rubber with 10-32% sulfur. This is a much higher sulfur concentration than is usually used in vulcanization and the process results in an extremely brittle, dense moldable product. Vulcanite is naturally dark brown and opaque, but on exposure to light and moisture, the sulfur on the surface can oxidize forming a yellowish to greenish tinge. This surface is also acidic and can corrode adjacent metals. When warmed, vulcanite exudes a sulfurous odor. It was used to make combs, buttons, brooches, boxes, medallions, pens, and pencils. Vulcanite had good electrical insulation properties and was widely used for insulation. | ||
| − | See also [[vulcanization|vulcanization]]. | + | See also [[vulcanization|vulcanization]] and [[vulcanized rubber]]. |
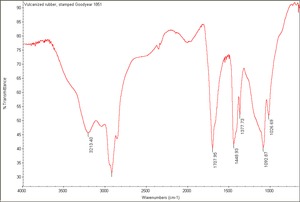
| + | [[[SliderGallery rightalign|Vulcanized rubber, stamped Goodyear 1851.TIF~FTIR(MFA)]]] | ||
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
Latest revision as of 15:16, 25 June 2022
Description
An old brand name for a hard, dark, shiny rubber developed in the 1830s. The term Vulcanite was used for marketing in the U.S. while the name Ebonite was used in England. Vulcanite is produced by heating natural rubber with 10-32% sulfur. This is a much higher sulfur concentration than is usually used in vulcanization and the process results in an extremely brittle, dense moldable product. Vulcanite is naturally dark brown and opaque, but on exposure to light and moisture, the sulfur on the surface can oxidize forming a yellowish to greenish tinge. This surface is also acidic and can corrode adjacent metals. When warmed, vulcanite exudes a sulfurous odor. It was used to make combs, buttons, brooches, boxes, medallions, pens, and pencils. Vulcanite had good electrical insulation properties and was widely used for insulation.
See also Vulcanization and Vulcanized rubber.
Synonyms and Related Terms
ebonite; vulcanita (Esp.);
Collection Risks
- Emits hydrogen sulfide and other sulfur containing gases upon degradation.
Physical and Chemical Properties
- Spot test for vulcanized rubber: Iodine/sodium azide reagent for presence of reducible sulfur compounds - positive reaction generates bubbles (Daniels and Ward, 1982)
- Density = 1.15 g/ml
Resources and Citations
- V.Daniels, S.Ward, "A Rapid Test for the Detection of Substances which will Tarnish Silver" Studies in Conservation 27:58-60, 1982.
- Plastics Museum Website
- Scott Williams, WAAC Newsletter 24(1) 2002 - Emits hydrogen sulfide and other sulfur containing gases upon degradation
- History of Plastic at www.nswpmith.com.au/historyofplastics.html
- The Dictionary of Art, Grove's Dictionaries Inc., New York, 1996 Comment: "Plastic"
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971
- Dictionary of Building Preservation, Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996
- Pam Hatchfield, Pollutants in the Museum Environment, Archetype Press, London, 2002
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980