Difference between revisions of "Vulcanized rubber"
(username removed) |
|||
| (3 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
| + | [[File:02.222-E11509CR-d1.jpg|thumb|Devotion panel<br>MFA# 02.222]] | ||
| + | == Description == | ||
| + | |||
| + | A hard, non-sticky, elastic form of rubber. In 1839, Charles Goodyear discovered a process, termed 'vulcanization' that makes natural rubber into a form that is elastic, flexible and heat resistant. Vulcanization was done by first heating rubber till it became a viscous mass, then sulfur compounds were added and the mixture cooled.. Vulcanized rubber is produced by heating natural rubber with 10-32% sulfur. This is a much higher sulfur concentration than is usually used in vulcanization and the process results in an extremely brittle, dense moldable product. Vulcanized rubber is naturally dark brown and opaque, but on exposure to light and moisture, the sulfur on the surface can oxidize forming a yellowish to greenish tinge. This surface is also acidic and can corrode adjacent metals. When warmed, vulcanized rubber exudes a sulfurous odor. It was used to make combs, buttons, brooches, boxes, medallions, pens, and pencils. It had good electrical insulation properties and was widely used for insulation. The term Vulcanite was used for marketing in the U.S. while the name [[Ebonite|Ebonite]] was used in England. | ||
| + | Vulcanization is a process used to harden and strengthen rubber. Discovered by in 1839, When vulcanized rubber is exposed to heat or light, it can degrade, forming a brownish, brittle material that may evolve sulfurous oxides. Copper containing alloys can accelerate oxidative degradation (Hatchfield 2002). Recently, a chemical vulcanization process has also been developed that occurs at room temperature. This room-temperature vulcanization (RTV) occurs when the rubber is mixed with a catalyst. The RTV hardening process is generally used with silicone rubbers. | ||
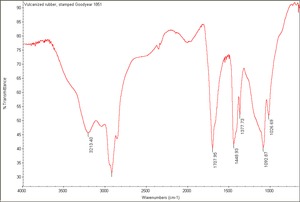
| + | [[[SliderGallery rightalign|Vulcanized rubber, stamped Goodyear 1851.TIF~FTIR(MFA)]]] | ||
| + | |||
| + | == Synonyms and Related Terms == | ||
| + | |||
| + | ebonite; vulcanita (Esp.); | ||
| + | |||
| + | == Collection Risks == | ||
| + | |||
| + | * Emits hydrogen sulfide and other sulfur containing gases upon degradation. | ||
| + | |||
| + | ==Physical and Chemical Properties== | ||
| + | |||
| + | * Spot test for vulcanized rubber: Iodine/sodium azide reagent for presence of reducible sulfur compounds - positive reaction generates bubbles (Daniels and Ward, 1982) | ||
| + | * Density = 1.15 g/ml | ||
| + | |||
| + | ==Resources and Citations== | ||
| + | |||
| + | * V.Daniels, S.Ward, "A Rapid Test for the Detection of Substances which will Tarnish Silver" ''Studies in Conservation'' 27:58-60, 1982. | ||
| + | |||
| + | * Plastics Museum [http://www.plastics-museum.com/ Website] | ||
| + | |||
| + | * Scott Williams, WAAC Newsletter 24(1) 2002 - Emits hydrogen sulfide and other sulfur containing gases upon degradation | ||
| + | |||
| + | * History of Plastic at www.nswpmith.com.au/historyofplastics.html | ||
| + | |||
| + | * ''The Dictionary of Art'', Grove's Dictionaries Inc., New York, 1996 Comment: "Plastic" | ||
| + | |||
| + | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 | ||
| + | |||
| + | * ''Dictionary of Building Preservation'', Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996 | ||
| + | |||
| + | * Pam Hatchfield, ''Pollutants in the Museum Environment'', Archetype Press, London, 2002 | ||
| + | |||
| + | * ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | ||
| + | |||
| + | * ''CRC Handbook of Chemistry and Physics'', Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 | ||
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 15:23, 25 June 2022
Description
A hard, non-sticky, elastic form of rubber. In 1839, Charles Goodyear discovered a process, termed 'vulcanization' that makes natural rubber into a form that is elastic, flexible and heat resistant. Vulcanization was done by first heating rubber till it became a viscous mass, then sulfur compounds were added and the mixture cooled.. Vulcanized rubber is produced by heating natural rubber with 10-32% sulfur. This is a much higher sulfur concentration than is usually used in vulcanization and the process results in an extremely brittle, dense moldable product. Vulcanized rubber is naturally dark brown and opaque, but on exposure to light and moisture, the sulfur on the surface can oxidize forming a yellowish to greenish tinge. This surface is also acidic and can corrode adjacent metals. When warmed, vulcanized rubber exudes a sulfurous odor. It was used to make combs, buttons, brooches, boxes, medallions, pens, and pencils. It had good electrical insulation properties and was widely used for insulation. The term Vulcanite was used for marketing in the U.S. while the name Ebonite was used in England.
Vulcanization is a process used to harden and strengthen rubber. Discovered by in 1839, When vulcanized rubber is exposed to heat or light, it can degrade, forming a brownish, brittle material that may evolve sulfurous oxides. Copper containing alloys can accelerate oxidative degradation (Hatchfield 2002). Recently, a chemical vulcanization process has also been developed that occurs at room temperature. This room-temperature vulcanization (RTV) occurs when the rubber is mixed with a catalyst. The RTV hardening process is generally used with silicone rubbers.
Synonyms and Related Terms
ebonite; vulcanita (Esp.);
Collection Risks
- Emits hydrogen sulfide and other sulfur containing gases upon degradation.
Physical and Chemical Properties
- Spot test for vulcanized rubber: Iodine/sodium azide reagent for presence of reducible sulfur compounds - positive reaction generates bubbles (Daniels and Ward, 1982)
- Density = 1.15 g/ml
Resources and Citations
- V.Daniels, S.Ward, "A Rapid Test for the Detection of Substances which will Tarnish Silver" Studies in Conservation 27:58-60, 1982.
- Plastics Museum Website
- Scott Williams, WAAC Newsletter 24(1) 2002 - Emits hydrogen sulfide and other sulfur containing gases upon degradation
- History of Plastic at www.nswpmith.com.au/historyofplastics.html
- The Dictionary of Art, Grove's Dictionaries Inc., New York, 1996 Comment: "Plastic"
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971
- Dictionary of Building Preservation, Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996
- Pam Hatchfield, Pollutants in the Museum Environment, Archetype Press, London, 2002
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980