Difference between revisions of "Whiting"
Jump to navigation
Jump to search
(username removed) |
|||
| (4 intermediate revisions by 3 users not shown) | |||
| Line 2: | Line 2: | ||
== Description == | == Description == | ||
| − | A fine powder of white [ | + | A fine powder of white [[chalk|chalk]] (native [[calcium%20carbonate|calcium carbonate]]). Whiting has been used as an inert pigment in paints, inks, and puttys and also as a flux in low temperature ceramic glazes. Synthetically prepared calcium carbonate, called precipitated chalk, is much whiter and finer than whiting. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 8: | Line 8: | ||
chalk; whitening; Spanish white; limestone whiting; Paris white; English white; gilder's whiting; Pigment White 18; CI 77220 | chalk; whitening; Spanish white; limestone whiting; Paris white; English white; gilder's whiting; Pigment White 18; CI 77220 | ||
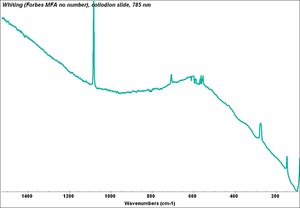
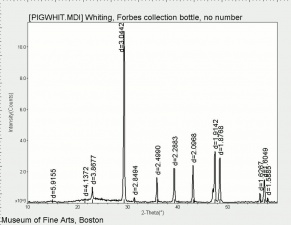
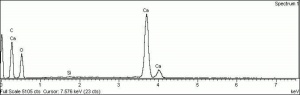
| − | [[[SliderGallery rightalign|PIGWHIT.jpg~XRD|fwhitingsem.jpg~SEM|fwhitingedsbw.jpg~EDS]]] | + | [[[SliderGallery rightalign|Whiting (Forbes MFA no number), collodion slide, 785 nm copy.tif~Raman (MFA)|PIGWHIT.jpg~XRD|fwhitingsem.jpg~SEM|fwhitingedsbw.jpg~EDS]]] |
| + | == Physical and Chemical Properties == | ||
| − | + | * Small irregular shaped particles (0.1-10 microns); | |
| − | + | * High birefringence with strong interference colors | |
| − | Small irregular shaped particles (0.1-10 microns); | + | * May fluoresce a medium purple color. |
| − | + | * Reacts with acids to evolve carbon dioxide. | |
| − | |||
{| class="wikitable" | {| class="wikitable" | ||
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 2.70 | + | | 2.70 g/ml |
|- | |- | ||
! scope="row"| Refractive Index | ! scope="row"| Refractive Index | ||
| Line 30: | Line 30: | ||
[[media:download_file_526.pdf|Characteristics of Common White Pigments]] | [[media:download_file_526.pdf|Characteristics of Common White Pigments]] | ||
| − | |||
| − | |||
== Additional Images == | == Additional Images == | ||
| Line 40: | Line 38: | ||
</gallery> | </gallery> | ||
| − | + | == Resources and Citations == | |
| − | == | ||
* Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, ''Pigment Compendium'', Elsevier Butterworth-Heinemann, Oxford, 2004 | * Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, ''Pigment Compendium'', Elsevier Butterworth-Heinemann, Oxford, 2004 | ||
| Line 57: | Line 54: | ||
* Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, ''Technology and Conservation'', Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985 | * Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, ''Technology and Conservation'', Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985 | ||
| − | * Art and Architecture Thesaurus Online, | + | * Art and Architecture Thesaurus Online, https://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 10:22, 27 June 2022
Description
A fine powder of white Chalk (native Calcium carbonate). Whiting has been used as an inert pigment in paints, inks, and puttys and also as a flux in low temperature ceramic glazes. Synthetically prepared calcium carbonate, called precipitated chalk, is much whiter and finer than whiting.
Synonyms and Related Terms
chalk; whitening; Spanish white; limestone whiting; Paris white; English white; gilder's whiting; Pigment White 18; CI 77220
Physical and Chemical Properties
- Small irregular shaped particles (0.1-10 microns);
- High birefringence with strong interference colors
- May fluoresce a medium purple color.
- Reacts with acids to evolve carbon dioxide.
| Density | 2.70 g/ml |
|---|---|
| Refractive Index | 1.486; 1.645 |
Comparisons
Properties of Common Abrasives
Characteristics of Common White Pigments
Additional Images
Resources and Citations
- Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, Pigment Compendium, Elsevier Butterworth-Heinemann, Oxford, 2004
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 181
- Reed Kay, The Painter's Guide To Studio Methods and Materials, Prentice-Hall, Inc., Englewood Cliffs, NJ, 1983
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- Paint in America, Robert Moss (ed.), John Wiley & Sons, New York, 1994 Comment: Ian Bristow "House Painting in Britain"
- Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985
- Art and Architecture Thesaurus Online, https://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000