Difference between revisions of "Rosaniline"
Jump to navigation
Jump to search
(username removed) |
|||
| (5 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
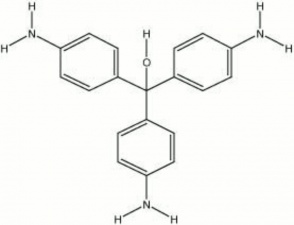
| − | Colorless needles and plates that forma a red solution. Rosaniline is formed from one molecule of [ | + | Colorless needles and plates that forma a red solution. Rosaniline is formed from one molecule of [[aniline|aniline]] and two of toluidine. All varieties of magenta dyes are salts this tripheylmethane compound. Chlorination of rosaniline produces [[magenta|magenta]] (rosaniline chloride). Currently, rosaniline is used as a [[fungicide|fungicide]], fabric [[dye|dye]], and [[ink|ink]] colorant. It is also a [[fluorochrome|fluorochrome]]. It has a mean excitation wavelength of 570 nm (green) and a mean emission wavelength of 625 nm (Wolbers et al. 1990). |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 9: | Line 9: | ||
[[[SliderGallery rightalign|rosaniline.jpg~Chemical structure]]] | [[[SliderGallery rightalign|rosaniline.jpg~Chemical structure]]] | ||
| − | == | + | == Physical and Chemical Properties == |
Soluble in acids, hot ethanol and aniline. Slightly soluble in boiling water. Insoluble in ether and benzene. | Soluble in acids, hot ethanol and aniline. Slightly soluble in boiling water. Insoluble in ether and benzene. | ||
| Line 22: | Line 22: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 205 | + | | 205 C |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 28: | Line 28: | ||
|} | |} | ||
| − | == | + | == Risks == |
| − | Potential carcinogen. Contact causes irritation. | + | * Potential carcinogen. |
| + | * Contact causes irritation. | ||
| + | * Fisher Scientific: [https://fscimage.fishersci.com/msds/86807.htm MSDS] | ||
| − | + | == Resources and Citations == | |
| − | + | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | |
| − | + | * Richard C. Wolbers, Nanette T. Sterman, Chris Stavroudis, ''Notes for Workshop on New Methods in the Cleaning of Paintings'', J.Paul Getty Trust, Los Angeles, 1990 | |
| − | |||
| − | |||
| − | |||
| − | * | ||
| − | |||
| − | |||
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 | ||
| − | * | + | * F. Crace-Calvert, ''Dyeing and Calico Printing'', Palmer & Howe, London, 1876 |
* Aldrich Chemical Catalog | * Aldrich Chemical Catalog | ||
Latest revision as of 14:29, 27 June 2022
Description
Colorless needles and plates that forma a red solution. Rosaniline is formed from one molecule of Aniline and two of toluidine. All varieties of magenta dyes are salts this tripheylmethane compound. Chlorination of rosaniline produces Magenta (rosaniline chloride). Currently, rosaniline is used as a Fungicide, fabric Dye, and Ink colorant. It is also a Fluorochrome. It has a mean excitation wavelength of 570 nm (green) and a mean emission wavelength of 625 nm (Wolbers et al. 1990).
Synonyms and Related Terms
roseaniline (sp); pararosaniline; rosaniline hydrochloride (magenta); CI 42500
Physical and Chemical Properties
Soluble in acids, hot ethanol and aniline. Slightly soluble in boiling water. Insoluble in ether and benzene.
| Composition | C20H19N3 |
|---|---|
| CAS | 25620-78-4 |
| Melting Point | 205 C |
| Molecular Weight | mol. wt. = 305.38 |
Risks
- Potential carcinogen.
- Contact causes irritation.
- Fisher Scientific: MSDS
Resources and Citations
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Richard C. Wolbers, Nanette T. Sterman, Chris Stavroudis, Notes for Workshop on New Methods in the Cleaning of Paintings, J.Paul Getty Trust, Los Angeles, 1990
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983
- F. Crace-Calvert, Dyeing and Calico Printing, Palmer & Howe, London, 1876
- Aldrich Chemical Catalog