Difference between revisions of "Rotenone"
Jump to navigation
Jump to search
m (Text replace - "\[http:\/\/cameo\.mfa\.org\/materials\/fullrecord\.asp\?name=([^\s]+)\s(.*)\]" to "$2") |
|||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | Odorless, colorless, crystals that are highly toxic to insects and fish but only | + | Odorless, colorless, crystals that are highly toxic to insects and fish but only mildly toxic to mammals. Rotenone naturally occurs in derris root and cubé. It was first isolated in 1895 by E. Geoffroy from the plant now called ''Lonchocarpus nicou''. This powdered plant was used to catch fish by poisoning them. The pesticide is still used to eradicate exotic fish from non-native habitats. Rotenone is also used as an [[insecticide|insecticide]], particularly in home gardens, due to its low toxicity to mammals. USDA allowed rotenone to be used on organic produce until 2005. |
| − | + | [[[SliderGallery rightalign|rotenone.jpg~Chemical structure]]] | |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
Tubatoxin; Prentox; Rotocide; Barbasco; Chem Fish; Cube; Deril | Tubatoxin; Prentox; Rotocide; Barbasco; Chem Fish; Cube; Deril | ||
| − | [ | + | == Risks == |
| + | |||
| + | * Combustibe. | ||
| + | * Decomposes on exposure to light and air. | ||
| + | * Ingestion, inhalation, and skin absorption causes irritation. | ||
| + | * Generally considered nontoxic to mammals but can cause death in large doses. | ||
| + | * Cayman Chemical: [https://cdn.caymanchem.com/cdn/msds/13995m.pdf SDS] | ||
| − | == | + | ==Physical and Chemical Properties== |
Soluble in alcohol, benzene, acetone, carbon tetrachloride, chloroform, ether. Insoluble in water. | Soluble in alcohol, benzene, acetone, carbon tetrachloride, chloroform, ether. Insoluble in water. | ||
| Line 22: | Line 28: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 165-166 | + | | 165-166 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 1.27 | + | | 1.27 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 31: | Line 37: | ||
|- | |- | ||
! scope="row"| Boiling Point | ! scope="row"| Boiling Point | ||
| − | | 210-220 | + | | 210-220 C |
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 8138 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 8138 | ||
| Line 48: | Line 46: | ||
* Random House, ''Webster's Encyclopedic Unabridged Dictionary of the English Language'', Grammercy Book, New York, 1997 | * Random House, ''Webster's Encyclopedic Unabridged Dictionary of the English Language'', Grammercy Book, New York, 1997 | ||
| − | * Wikipedia | + | * Wikipedia: Rotenone (accessed Jan. 9 2009) |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 07:37, 28 June 2022
Description
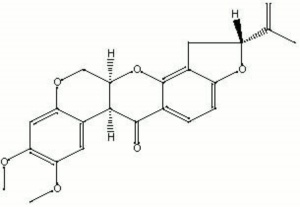
Odorless, colorless, crystals that are highly toxic to insects and fish but only mildly toxic to mammals. Rotenone naturally occurs in derris root and cubé. It was first isolated in 1895 by E. Geoffroy from the plant now called Lonchocarpus nicou. This powdered plant was used to catch fish by poisoning them. The pesticide is still used to eradicate exotic fish from non-native habitats. Rotenone is also used as an Insecticide, particularly in home gardens, due to its low toxicity to mammals. USDA allowed rotenone to be used on organic produce until 2005.
Synonyms and Related Terms
Tubatoxin; Prentox; Rotocide; Barbasco; Chem Fish; Cube; Deril
Risks
- Combustibe.
- Decomposes on exposure to light and air.
- Ingestion, inhalation, and skin absorption causes irritation.
- Generally considered nontoxic to mammals but can cause death in large doses.
- Cayman Chemical: SDS
Physical and Chemical Properties
Soluble in alcohol, benzene, acetone, carbon tetrachloride, chloroform, ether. Insoluble in water.
| Composition | C23H22O6 |
|---|---|
| CAS | 83-79-4 |
| Melting Point | 165-166 C |
| Density | 1.27 g/ml |
| Molecular Weight | mol. wt. = 394.4 |
| Boiling Point | 210-220 C |
Resources and Citations
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 8138
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- Wikipedia: Rotenone (accessed Jan. 9 2009)