Difference between revisions of "Dimethylformamide"
Jump to navigation
Jump to search
| (One intermediate revision by one other user not shown) | |||
| Line 2: | Line 2: | ||
A clear, toxic liquid that is considered a universal [[solvent]] since it is miscible in both water and organic solvents. Dimethylformamide (DMF) dissolves most natural and synthetic resins, and, with heat and time, DMF will even soften [[epoxy]]. It is often used in liquid chromatographic systems as a gradient intermediate between polar and nonpolar solvents. DMF is also used in some commercial paint stripping formulations. | A clear, toxic liquid that is considered a universal [[solvent]] since it is miscible in both water and organic solvents. Dimethylformamide (DMF) dissolves most natural and synthetic resins, and, with heat and time, DMF will even soften [[epoxy]]. It is often used in liquid chromatographic systems as a gradient intermediate between polar and nonpolar solvents. DMF is also used in some commercial paint stripping formulations. | ||
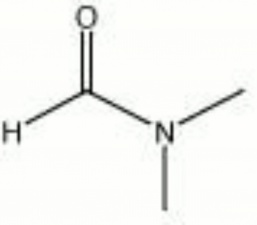
| − | + | [[[SliderGallery rightalign|dimethylformamide.jpg~Chemical structure]]] | |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
DMF; DMFA; n,n-dimethyl formamide | DMF; DMFA; n,n-dimethyl formamide | ||
| − | [ | + | == Risks == |
| + | |||
| + | * Carcinogenic. | ||
| + | * Overexposure may cause vomiting, liver damage and high blood pressure. | ||
| + | * Irritant to skin. | ||
| + | * Combustible; moderate fire risk. Flash point = 58C | ||
| + | * Toxic by inhalation, ingestion and skin absorption. | ||
| + | * Greenfield: [https://greenfield.com/wp-content/uploads/2018/11/DIMETHYLFORMAMIDE-DMF-SDS_US-English.pdf SDS] | ||
| − | == | + | ==Physical and Chemical Properties== |
Miscible with water and most common organic solvents. | Miscible with water and most common organic solvents. | ||
| Line 22: | Line 29: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | -61 | + | | -61 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 0.9445 | + | | 0.9445 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 31: | Line 38: | ||
|- | |- | ||
! scope="row"| Boiling Point | ! scope="row"| Boiling Point | ||
| − | | 153 | + | | 153 C |
|} | |} | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
== Comparisons == | == Comparisons == | ||
| Line 46: | Line 45: | ||
[[media:download_file_125.pdf|Properties of Common Solvents]] | [[media:download_file_125.pdf|Properties of Common Solvents]] | ||
| − | + | ==Resources and Citations== | |
| − | |||
| − | == | ||
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 9232 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 9232 | ||
Latest revision as of 14:05, 21 July 2022
Description
A clear, toxic liquid that is considered a universal Solvent since it is miscible in both water and organic solvents. Dimethylformamide (DMF) dissolves most natural and synthetic resins, and, with heat and time, DMF will even soften Epoxy. It is often used in liquid chromatographic systems as a gradient intermediate between polar and nonpolar solvents. DMF is also used in some commercial paint stripping formulations.
Synonyms and Related Terms
DMF; DMFA; n,n-dimethyl formamide
Risks
- Carcinogenic.
- Overexposure may cause vomiting, liver damage and high blood pressure.
- Irritant to skin.
- Combustible; moderate fire risk. Flash point = 58C
- Toxic by inhalation, ingestion and skin absorption.
- Greenfield: SDS
Physical and Chemical Properties
Miscible with water and most common organic solvents.
| Composition | HCON(CH3)2 |
|---|---|
| CAS | 68-12-2 |
| Melting Point | -61 C |
| Density | 0.9445 g/ml |
| Molecular Weight | mol. wt. = 73.09 |
| Boiling Point | 153 C |
Comparisons
Resources and Citations
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 9232
- Hermann Kuhn, Conservation and Restoration of Works of Art and Antiquities, Butterworths, London, 1986