Difference between revisions of "Henna"
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
|||
| (One intermediate revision by the same user not shown) | |||
| Line 9: | Line 9: | ||
[[[SliderGallery rightalign|henna.jpg~Chemical structure]]] | [[[SliderGallery rightalign|henna.jpg~Chemical structure]]] | ||
| + | == Risks == | ||
| − | == | + | * Contact, inhalation or ingestion may cause irritation. |
| + | * Fisher Scientific: [https://fscimage.fishersci.com/msds/97034.htm MSDS] | ||
| + | |||
| + | == Physical and Chemical Properties == | ||
Lawsone is soluble in water, ether. | Lawsone is soluble in water, ether. | ||
| Line 29: | Line 33: | ||
|} | |} | ||
| − | == | + | == Resources and Citations == |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | * J.Hofenk-de Graaf, ''Natural Dyestuffs: Origin, Chemical Constitution, Identification'', Central Research Laboratory for Objects of Art and Science, Amsterdam, September 1969. | |
| − | |||
| − | |||
* ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | * ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | ||
| Line 51: | Line 47: | ||
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
| − | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: "Myrtales" | + | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: "Myrtales" [Accessed May 6, 2002]. ''Lawsonia inermis'' |
* Palmy Weigle, ''Ancient Dyes for Modern Weavers'', Watson-Guptill Publications, New York, 1974 | * Palmy Weigle, ''Ancient Dyes for Modern Weavers'', Watson-Guptill Publications, New York, 1974 | ||
| Line 60: | Line 56: | ||
* F. Crace-Calvert, ''Dyeing and Calico Printing'', Palmer & Howe, London, 1876 | * F. Crace-Calvert, ''Dyeing and Calico Printing'', Palmer & Howe, London, 1876 | ||
| − | |||
| − | |||
* J. Thornton, 'The Use of Dyes and Colored Varnishes in Wood Polychromy', ''Painted Wood: History and Conservation'', The Getty Conservation Insitute, Los Angeles, 1998 | * J. Thornton, 'The Use of Dyes and Colored Varnishes in Wood Polychromy', ''Painted Wood: History and Conservation'', The Getty Conservation Insitute, Los Angeles, 1998 | ||
Latest revision as of 07:37, 9 August 2022
Description
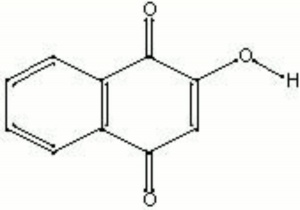
A reddish-orange dyestuff that comes from the leaves of small evergreen trees, Lawsonia inermis or Lawsonia alba, native to the Middle East, northern Africa and Asia. Henna was used by the ancient Egyptians and Asians for dyeing Hair, nails, skin, Leather, Wool, and Silk. Henna-dyed cloth was sometimes used for mummy wrappings. The principle colorant in henna is lawsone and it also contains Juglone, Luteolin, and several tannins. Lawsone is a red Substantive dye that is very effective on the protein Keratin. Henna has also been used as a Fungicide.
Synonyms and Related Terms
2-hydroxy-1,4-naphthalenedione; Natural Orange 6; CI 75480; henna (Esp.); henné (Fr.); hena (Port.); English privet; flowers of paradise; lawsone; 2-hydroxy-p-naphthoquinone; 2-hydroxynaphthoquinone; hennis; hana; alkanna; gopherwood; mignonette; mehandi; mendi
Risks
- Contact, inhalation or ingestion may cause irritation.
- Fisher Scientific: MSDS
Physical and Chemical Properties
Lawsone is soluble in water, ether.
| Composition | C10H6O3 |
|---|---|
| CAS | 83-72-7 |
| Melting Point | 192 (dec) |
| Molecular Weight | mol. wt. = 174.0402 |
Resources and Citations
- J.Hofenk-de Graaf, Natural Dyestuffs: Origin, Chemical Constitution, Identification, Central Research Laboratory for Objects of Art and Science, Amsterdam, September 1969.
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 283
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Encyclopedia Britannica, http://www.britannica.com Comment: "Myrtales" [Accessed May 6, 2002]. Lawsonia inermis
- Palmy Weigle, Ancient Dyes for Modern Weavers, Watson-Guptill Publications, New York, 1974
- John and Margaret Cannon, Dye Plants and Dyeing, Herbert Press, London, 1994
- A.Lucas, J.R.Harris, Ancient Egyptian Materials and Industries, Edward Arnold Publishers Ltd., London, 4th edition, 1962
- F. Crace-Calvert, Dyeing and Calico Printing, Palmer & Howe, London, 1876
- J. Thornton, 'The Use of Dyes and Colored Varnishes in Wood Polychromy', Painted Wood: History and Conservation, The Getty Conservation Insitute, Los Angeles, 1998
- Helmut Schweppe, Schweppe color collection index and information book
- Colour Index International online at www.colour-index.org