Difference between revisions of "Methylene blue"
(username removed) |
|||
| (2 intermediate revisions by one other user not shown) | |||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | Dark green crystals that form a deep blue aqueous solution. Methylene blue was first prepared in 1876 by Caro. It is a synthetic [ | + | Dark green crystals that form a deep blue aqueous solution. Methylene blue was first prepared in 1876 by Caro. It is a synthetic |
| − | + | [[aniline%20dye|aniline dye]] that was used a lightfast colorant for | |
| + | [[cotton|cotton]], | ||
| + | [[silk|silk]], and | ||
| + | [[wool|wool]] (often with zinc as a mordant). Methylene blue is used as a biological stain for bacteria and as an indicator in oxidation-reduction reactions. Methylene blue is also used as a | ||
| + | [[disinfectant|disinfectant]] and an antidote for | ||
| + | [[sodium%20cyanide|cyanide]] poisoning. | ||
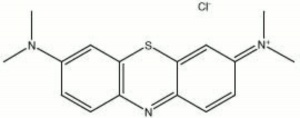
| + | [[[SliderGallery rightalign|methylene blue.jpg~Chemical structure]]] | ||
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
methylthionine chloride; Basic Blue 9; CI 52015; Solvent Blue 8; 3,7-bis(dimethylamino)phenothiazin-5-ium chloride; azul de metileno (Esp.); azul de metileno (Port.); methyl blue; solvent blue; Basic Lake Blue; Swiss blue | methylthionine chloride; Basic Blue 9; CI 52015; Solvent Blue 8; 3,7-bis(dimethylamino)phenothiazin-5-ium chloride; azul de metileno (Esp.); azul de metileno (Port.); methyl blue; solvent blue; Basic Lake Blue; Swiss blue | ||
| − | [ | + | ==Risks == |
| + | * Toxic by ingestion and inhalation. | ||
| + | * Contact may cause irritation. | ||
| + | * Potential teratogen. | ||
| + | * Flammable. Flash point = 178 C | ||
| + | * Fisher Scientific: [https://fscimage.fishersci.com/msds/96419.htm MSDS] | ||
| − | == | + | ==Physical and Chemical Properties== |
Soluble in water, ethanol, chloroform. pH of aq. solution = 3 - 4.5 | Soluble in water, ethanol, chloroform. pH of aq. solution = 3 - 4.5 | ||
| Line 24: | Line 35: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 190 (dec) | + | | 190 C (dec) |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 30: | Line 41: | ||
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
Latest revision as of 13:41, 1 October 2022
Description
Dark green crystals that form a deep blue aqueous solution. Methylene blue was first prepared in 1876 by Caro. It is a synthetic
Aniline dye that was used a lightfast colorant for
Cotton,
Silk, and
Wool (often with zinc as a mordant). Methylene blue is used as a biological stain for bacteria and as an indicator in oxidation-reduction reactions. Methylene blue is also used as a
Disinfectant and an antidote for
cyanide poisoning.
Synonyms and Related Terms
methylthionine chloride; Basic Blue 9; CI 52015; Solvent Blue 8; 3,7-bis(dimethylamino)phenothiazin-5-ium chloride; azul de metileno (Esp.); azul de metileno (Port.); methyl blue; solvent blue; Basic Lake Blue; Swiss blue
Risks
- Toxic by ingestion and inhalation.
- Contact may cause irritation.
- Potential teratogen.
- Flammable. Flash point = 178 C
- Fisher Scientific: MSDS
Physical and Chemical Properties
Soluble in water, ethanol, chloroform. pH of aq. solution = 3 - 4.5
Insoluble in ether.
| Composition | C16H18N3SCl-3H2O |
|---|---|
| CAS | 61-73-4 |
| Melting Point | 190 C (dec) |
| Molecular Weight | mol. wt. = 319.85 |
Resources and Citations
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 6137
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- A.Scharff, 'Synthetic dyestuffs for textiles and their fastness to washing', ICOM-CC Preprints Lyon, Getty Conservation Institute, Los Angeles, 1999
- Colour Index International online at www.colour-index.org