Difference between revisions of "Magnesium carbonate, basic"
Jump to navigation
Jump to search
(username removed) |
|||
| (3 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | A white friable powder that has similar properties to [ | + | A white friable powder that has similar properties to [[magnesium%20carbonate|magnesium carbonate]]. |
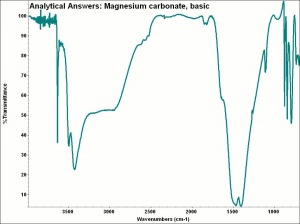
| − | + | [[[SliderGallery rightalign|aaiMGCO3basic.jpg~FTIR]]] | |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
magnesium carbonate hydroxide; magnesia alba | magnesium carbonate hydroxide; magnesia alba | ||
| − | [ | + | == Risks == |
| + | |||
| + | * Nontoxic. | ||
| + | * Ingestion has a laxative effect. | ||
| + | * Noncombustible. | ||
| + | * American Elements: [https://www.americanelements.com/magnesium-carbonate-basic-12125-28-9 SDS] | ||
| − | == | + | ==Physical and Chemical Properties== |
Soluble in acids. Slightly soluble in water. Insoluble in ethanol. | Soluble in acids. Slightly soluble in water. Insoluble in ethanol. | ||
| Line 19: | Line 24: | ||
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
Latest revision as of 12:35, 16 October 2022
Description
A white friable powder that has similar properties to Magnesium carbonate.
Synonyms and Related Terms
magnesium carbonate hydroxide; magnesia alba
Risks
- Nontoxic.
- Ingestion has a laxative effect.
- Noncombustible.
- American Elements: SDS
Physical and Chemical Properties
Soluble in acids. Slightly soluble in water. Insoluble in ethanol.
| Composition | Mg(OH)2-3MgCO3-3H2O |
|---|
Resources and Citations
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 5696