Difference between revisions of "Malathion"
(username removed) |
|||
| (3 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
| − | [[File: | + | [[File:malathionms.jpg|thumb|Mass spectrum of malathion]] |
== Description == | == Description == | ||
| − | A dark yellow liquid that is used as an [ | + | A dark yellow liquid that is used as an [[insecticide|insecticide]]. Malathion is an organophosphate compound that acts as a cholinesterase inhibitor. It has an unpleasant, [[sulfur|sulfur]] type odor and can be corrosive to [[iron|iron]]. Malathion has been used for control of [[cockroach|cockroaches]], treatment of head lice, and the aerial spraying to eradicate the Mediterranean fruit fly. Malathion biodegrades to form nontoxic compounds. |
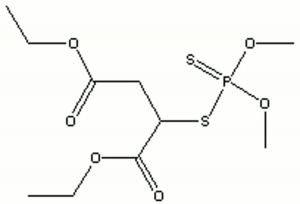
| + | [[[SliderGallery rightalign|malathionir.jpg~FTIR|malathionstr.jpg~Chemical structure]]] | ||
| − | |||
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
S-[1,2-bis(ethoxycarbonyl)ethyl]O,O-dimethylphosphorodithioate; carbofos; mercaptothion; [(dimethoxyphosphinothioyl)thio]butanedioic acid diethyl ester; insecticide no. 4049; Cythion [American Cyanamid] | S-[1,2-bis(ethoxycarbonyl)ethyl]O,O-dimethylphosphorodithioate; carbofos; mercaptothion; [(dimethoxyphosphinothioyl)thio]butanedioic acid diethyl ester; insecticide no. 4049; Cythion [American Cyanamid] | ||
| − | + | == Risks == | |
| − | == | + | * Relatively low toxicity for mammals. |
| + | * Overexposure through inhalation and skin absorption can causes irritation and/or vision/breathing problems. LD50=2800 mg/kg | ||
| + | * Potential teratogen. | ||
| + | * Combustible. | ||
| + | * Corrodes iron, steel, tin, lead, and copper. May discolor organic red dyes. | ||
| + | * WinField: [http://www.cdms.net/ldat/mp41D000.pdf SDS] | ||
| + | |||
| + | == Physical and Chemical Properties == | ||
Miscible with alcohols, ketones, ether, aromatic hydrocarbons and oils. Slightly soluble in water. Decomposes with acids or bases to produce sulfur compounds. | Miscible with alcohols, ketones, ether, aromatic hydrocarbons and oils. Slightly soluble in water. Decomposes with acids or bases to produce sulfur compounds. | ||
| Line 24: | Line 31: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 2.9 | + | | 2.9 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 1.23 | + | | 1.23 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 33: | Line 40: | ||
|- | |- | ||
! scope="row"| Boiling Point | ! scope="row"| Boiling Point | ||
| − | | 156-157 | + | | 156-157 C |
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | + | * Lynda A. Zycherman, J.Richard Schrock, ''A Guide to Museum Pest Control'', FAIC and Association of Systematics Collections, Washington DC, 1988 | |
| − | + | * J. Dawson, ''CCI Technical Bulletin'', 'Solving Museum Insect Problems: Chemical Control' , Canadian Conservation Institute, Ottawa, No. 15 | |
| − | + | == Sources Checked for Data in Record == | |
| − | + | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | * | ||
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry # 5740 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry # 5740 | ||
| − | * | + | * Pam Hatchfield, ''Pollutants in the Museum Environment'', Archetype Press, London, 2002 |
| − | |||
| − | |||
| − | |||
| − | |||
| − | * | + | * G.Caneva, M.P.Nugari, O.Salvadori, ''Biology in the Conservation of Works of Art'', ICCROM, Rome, 1991 |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 13:51, 16 October 2022
Description
A dark yellow liquid that is used as an Insecticide. Malathion is an organophosphate compound that acts as a cholinesterase inhibitor. It has an unpleasant, Sulfur type odor and can be corrosive to Iron. Malathion has been used for control of cockroaches, treatment of head lice, and the aerial spraying to eradicate the Mediterranean fruit fly. Malathion biodegrades to form nontoxic compounds.
Synonyms and Related Terms
S-[1,2-bis(ethoxycarbonyl)ethyl]O,O-dimethylphosphorodithioate; carbofos; mercaptothion; [(dimethoxyphosphinothioyl)thio]butanedioic acid diethyl ester; insecticide no. 4049; Cythion [American Cyanamid]
Risks
- Relatively low toxicity for mammals.
- Overexposure through inhalation and skin absorption can causes irritation and/or vision/breathing problems. LD50=2800 mg/kg
- Potential teratogen.
- Combustible.
- Corrodes iron, steel, tin, lead, and copper. May discolor organic red dyes.
- WinField: SDS
Physical and Chemical Properties
Miscible with alcohols, ketones, ether, aromatic hydrocarbons and oils. Slightly soluble in water. Decomposes with acids or bases to produce sulfur compounds.
| Composition | C10H19O6PS2 |
|---|---|
| CAS | 121-75-5 |
| Melting Point | 2.9 C |
| Density | 1.23 g/ml |
| Molecular Weight | mol. wt. = 330.4 |
| Boiling Point | 156-157 C |
Resources and Citations
- Lynda A. Zycherman, J.Richard Schrock, A Guide to Museum Pest Control, FAIC and Association of Systematics Collections, Washington DC, 1988
- J. Dawson, CCI Technical Bulletin, 'Solving Museum Insect Problems: Chemical Control' , Canadian Conservation Institute, Ottawa, No. 15
Sources Checked for Data in Record
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry # 5740
- Pam Hatchfield, Pollutants in the Museum Environment, Archetype Press, London, 2002
- G.Caneva, M.P.Nugari, O.Salvadori, Biology in the Conservation of Works of Art, ICCROM, Rome, 1991