Difference between revisions of "Methyl methacrylate"
Jump to navigation
Jump to search
m (Text replace - "\[http:\/\/cameo\.mfa\.org\/materials\/fullrecord\.asp\?name=([^\s]+)\s(.*)\]" to " $2") |
|||
| Line 7: | Line 7: | ||
methacrylic acid methyl ester; methyl 2-methylpropenoate; metacrilato de metilo (Esp.); 2-metilpropenoato de metilo (Esp.); méthylméthacrylate (Fr.); metilmetacrilato (It.); acrilato de metilo (Port.) | methacrylic acid methyl ester; methyl 2-methylpropenoate; metacrilato de metilo (Esp.); 2-metilpropenoato de metilo (Esp.); méthylméthacrylate (Fr.); metilmetacrilato (It.); acrilato de metilo (Port.) | ||
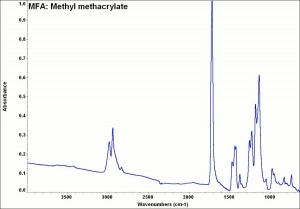
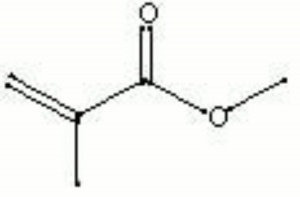
| + | [[[SliderGallery rightalign|MFA- Methyl methacrylate.jpg~FTIR|methyl methacrylate.jpg~Chemical structure]]] | ||
| − | + | == Risks == | |
| − | == | + | * Highly flammable. Flash point = 10 C. Explosive limits in air 2.1-12.5%. |
| + | * Hazardous by ingestion, inhalation and skin absorption. | ||
| + | * Contact causes irritation. | ||
| + | * ThermoFisher; [https://www.fishersci.com/store/msds?partNumber=AC127140100&productDescription=METHYL+METHACRYLATE+STA+10LTM&vendorId=VN00032119&countryCode=US&language=en SDS] | ||
| + | == Physical and Chemical Properties == | ||
Soluble in most organic solvents. Slightly soluble in water. | Soluble in most organic solvents. Slightly soluble in water. | ||
| Line 23: | Line 28: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | -48 | + | | -48 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 0.940 | + | | 0.940 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 35: | Line 40: | ||
|- | |- | ||
! scope="row"| Boiling Point | ! scope="row"| Boiling Point | ||
| − | | 100-101 | + | | 100-101 C |
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 | * R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 | ||
Revision as of 14:59, 18 October 2022
Description
A colorless, volatile liquid that is the monomer for Polymethyl methacrylate resins. Methyl methacrylate forms a clear glasslike, thermoplastic polymer that is light weight but hard to break. Methyl methacrylate polymerizes with heat, light, ionizing radiation, or catalysts. It can be copolymerized with many other monomers including other acrylate and methacrylate esters.
Synonyms and Related Terms
methacrylic acid methyl ester; methyl 2-methylpropenoate; metacrilato de metilo (Esp.); 2-metilpropenoato de metilo (Esp.); méthylméthacrylate (Fr.); metilmetacrilato (It.); acrilato de metilo (Port.)
Risks
- Highly flammable. Flash point = 10 C. Explosive limits in air 2.1-12.5%.
- Hazardous by ingestion, inhalation and skin absorption.
- Contact causes irritation.
- ThermoFisher; SDS
Physical and Chemical Properties
Soluble in most organic solvents. Slightly soluble in water.
| Composition | CH2:C(CH3)COOCH3 |
|---|---|
| CAS | 80-62-6 |
| Melting Point | -48 C |
| Density | 0.940 g/ml |
| Molecular Weight | mol. wt. = 100.1 |
| Refractive Index | 1.482-1.521 |
| Boiling Point | 100-101 C |
Resources and Citations
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 10
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Theodore J. Reinhart, 'Glossary of Terms', Engineered Plastics, ASM International, 1988
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997