Difference between revisions of "Morpholine"
(username removed) |
|||
| (2 intermediate revisions by one other user not shown) | |||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | A colorless, [ | + | A colorless, [[hygroscopic|hygroscopic]] liquid with a characteristic amine odor. Morpholine is miscible with water, but it evolves heat in the process forming a basic solution that can saponify dried oil films and aid in their removal. Morpholine is also used to dissolve [[resin|resins]], [[wax|waxes]], [[casein|casein]], [[shellac|shellac]], and [[dye|dyes]]. It can also act as a [[surfactant|surfactant]] and [[emulsifier|emulsifier]]. Morpholine has been used as a corrosion inhibitor in fire sprinkler and HVAC systems. It has also been used as a vapor phase neutralizing/alkalizing agent, but it is not recommended because of its toxicity, disagreeable odor, and poor ability to provide residual alkalinity. Some materials treated with morpholine, such as [[leather|leather]] and [[pyroxylin|pyroxylin]] coated book covers, have exhibited color changes (Book and Paper Catalog). |
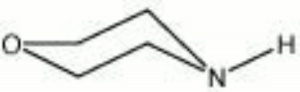
| − | + | [[[SliderGallery rightalign|morpholine.jpg~Chemical structure]]] | |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
tetrahydro-1,4-oxazine; diethylene oximide; diethylene imidoxide | tetrahydro-1,4-oxazine; diethylene oximide; diethylene imidoxide | ||
| − | + | == Risks == | |
| − | == | + | * Flammable, moderate fire risk. Flash point = 38C |
| + | * Toxic by inhalation, ingestion and skin absorption. | ||
| + | * Skin contact causes irritation. | ||
| + | * ThermoFisher: [https://www.fishersci.com/store/msds?partNumber=AC415160050&productDescription=MORPHOLINE+REAGENT+ACS+99%25+5G&vendorId=VN00032119&countryCode=US&language=en SDS] | ||
| + | == Physical and Chemical Properties == | ||
Soluble in water and organic solvents. | Soluble in water and organic solvents. | ||
| Line 24: | Line 28: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | -4.9 | + | | -4.9 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 1.007 | + | | 1.007 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 33: | Line 37: | ||
|- | |- | ||
! scope="row"| Boiling Point | ! scope="row"| Boiling Point | ||
| − | | 128.9 | + | | 128.9 C |
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
Latest revision as of 08:43, 19 October 2022
Description
A colorless, Hygroscopic liquid with a characteristic amine odor. Morpholine is miscible with water, but it evolves heat in the process forming a basic solution that can saponify dried oil films and aid in their removal. Morpholine is also used to dissolve resins, waxes, Casein, Shellac, and dyes. It can also act as a Surfactant and Emulsifier. Morpholine has been used as a corrosion inhibitor in fire sprinkler and HVAC systems. It has also been used as a vapor phase neutralizing/alkalizing agent, but it is not recommended because of its toxicity, disagreeable odor, and poor ability to provide residual alkalinity. Some materials treated with morpholine, such as Leather and Pyroxylin coated book covers, have exhibited color changes (Book and Paper Catalog).
Synonyms and Related Terms
tetrahydro-1,4-oxazine; diethylene oximide; diethylene imidoxide
Risks
- Flammable, moderate fire risk. Flash point = 38C
- Toxic by inhalation, ingestion and skin absorption.
- Skin contact causes irritation.
- ThermoFisher: SDS
Physical and Chemical Properties
Soluble in water and organic solvents.
Vapor pressure = 8.0 at 20C
| Composition | C4H8ONH |
|---|---|
| CAS | 110-91-8 |
| Melting Point | -4.9 C |
| Density | 1.007 g/ml |
| Molecular Weight | mol. wt. = 87.1 |
| Boiling Point | 128.9 C |
Resources and Citations
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 6362
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982
- Hermann Kuhn, Conservation and Restoration of Works of Art and Antiquities, Butterworths, London, 1986
- Pam Hatchfield, Pollutants in the Museum Environment, Archetype Press, London, 2002
- Book and Paper Group, Paper Conservation Catalog, AIC, 1984, 1989