Difference between revisions of "Benzoic acid"
Jump to navigation
Jump to search
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | A white, crystalline powder obtained from [[benzoin resin]] or other [[balsam|balsams]]. Benzoic acid is used as a mordant in calico printing, as an antifungal agent and for organic synthetic. Small amounts of benzoic acid may evolve from degraded plasticizers in [[polyvinyl chloride]] ( | + | A white, crystalline powder obtained from [[benzoin resin]] or other [[balsam|balsams]]. Benzoic acid is used as a mordant in calico printing, as an antifungal agent and for organic synthetic. Small amounts of benzoic acid may evolve from degraded plasticizers in [[polyvinyl chloride]] (Tétreault 2017). |
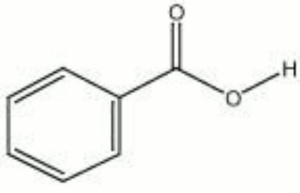
[[[SliderGallery rightalign|benzoic acid.jpg~Chemical structure]]] | [[[SliderGallery rightalign|benzoic acid.jpg~Chemical structure]]] | ||
Latest revision as of 12:39, 20 November 2022
Description
A white, crystalline powder obtained from Benzoin resin or other balsams. Benzoic acid is used as a mordant in calico printing, as an antifungal agent and for organic synthetic. Small amounts of benzoic acid may evolve from degraded plasticizers in Polyvinyl chloride (Tétreault 2017).
Synonyms and Related Terms
acid of benzoin; carboxybenzene; benzenecarboxylic acid; phenyl carboxylic acid; phenylformic acid; dracylic acid
Personal Risks
- Mildly irritating to skin and membranes.
- Moderately toxic by ingestion.
- Combustible.
- Fisher Scientific: SDS
Collection Risks
- Can corrode copper and bronze
Physical and Chemical Properties
Soluble in ethanol, ether, chloroform, benzene, carbon disulfide, carbon tetrachloride, turpentine. Slightly soluble in water.
| Composition | C6H5COOH |
|---|---|
| CAS | 65-85-0 |
| Melting Point | 122.4 C |
| Density | 1.2659 g/ml |
| Molecular Weight | mol. wt.=122.1 |
| Boiling Point | 249.2 C |
Resources and Citations
- Jean Tétreault, 'Products used in Preventive Conservation' Technical Bulletin #2, CCI, 2017. Link
- Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 1122
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 777
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998