Difference between revisions of "Polyethylene"
| (7 intermediate revisions by the same user not shown) | |||
| Line 4: | Line 4: | ||
== Description == | == Description == | ||
| − | A thermoplastic | + | A thermoplastic polymer in the polyolefin family with a base formula of [-CH2-]n. Ethylene was first polymerized in 1933 by ICI in England and was commercially released as Alkathene in 1939. In 1954, Karl Ziegler developed a process for high molecular weight polyethylene that allowed it to be spun into fibers, molded into durable but flexible forms and cast as tough thin sheets. Polyethylene made by the original process is now called [[low density polyethylene]] (LDPE; density = 0.92, melting pt=110-120 C) because if has extensive branching resulting in a softer, more flexible product with low tensile strength. Polymers made by the later Ziegler or Phillips processes are called [[high density polyethylene]] or (HDPE; density=0.95-0.96, melting pt=130-138C) because they lave a low degree of branching resulting in high tensile strength. LDPE is softer, more flexible and has of lower tensile strength than HDPE <ref name=T>Tetreault, Jean. Products Used in Preventative Conservation- Technical Bulletin 32. https://www.canada.ca/en/conservation-institute/services/conservation-preservation-publications/technical-bulletins/products-used-preventive-conservation.html#a3b1e</ref>, <ref name=Sh>Shashoua, Yvonne. Conservation of Plastics: Materials Science, Degradation and Preservation. Amsterdam etc.: Elsevier, 2008.</ref>. HDPE tends to have a longer lifespan than LDPE <ref name=T/>. In general, polyethylenes are translucent waxy polymers with good impact strength that are widely used for packaging, coatings, liners, plastic sheets, wire coatings, underwater cables, containers, waste bags, toys, and squeeze bottles. Small amounts of additives (antioxidants, light stabilizers, slip agents, antistatic agents, flame retardants, pigments, etc.) are typically added to the final products. Polyethylene is recyclable and many products, such as [[Tyvek]], contain the recycled polymer. |
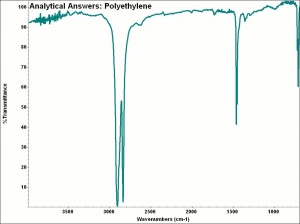
| − | + | [[[SliderGallery rightalign|aaiP-ETHYLN.jpg~FTIR]]] | |
Examples include: | Examples include: | ||
{| class="wikitable" style="text-align:center" | {| class="wikitable" style="text-align:center" | ||
| Line 13: | Line 13: | ||
|Closed cell foam | |Closed cell foam | ||
(non-crosslinked) | (non-crosslinked) | ||
| − | |Sealed Air Corp. formerly Dow; Ethafoam 180, 220, 400, 600, 900, 180 AS, 220 AS<br> | + | |Sealed Air Corp. formerly Dow; [[Ethafoam]] 180, 220, 400, 600, 900, 180 AS, 220 AS<br> |
| − | Pregis Corp; PolyPlank | + | Pregis Corp; [[PolyPlank]] |
|- | |- | ||
! High-Density Polyethylene (HDPE) | ! High-Density Polyethylene (HDPE) | ||
|Closed-cell foam (cross-linked) | |Closed-cell foam (cross-linked) | ||
| − | |Sekisui Voltek; Volara | + | |Sekisui Voltek; [[Volara]] |
| − | Zotefoams Ltd.; Plastazote | + | Zotefoams Ltd.; [[Plastazote]] |
MicroCell | MicroCell | ||
|- | |- | ||
! High-Density Polyethylene (HDPE) | ! High-Density Polyethylene (HDPE) | ||
|Spun bonded fiber | |Spun bonded fiber | ||
| − | |DuPont; Tyvek-10(stiff), Tyvek-14(soft), <br>Tyvek-16(perforated) | + | |DuPont; [[Tyvek]]-10(stiff), Tyvek-14(soft), <br>Tyvek-16(perforated) |
|- | |- | ||
!Low-Density Polyethylene (LDPE)<br> | !Low-Density Polyethylene (LDPE)<br> | ||
| Line 32: | Line 32: | ||
!Low-Density Polyethylene (LDPE) | !Low-Density Polyethylene (LDPE) | ||
|Plastic film with sealed bubbles | |Plastic film with sealed bubbles | ||
| − | |Sealed Air Corp (Bubble Wrap, Polycap, Aircap)<br> | + | |Sealed Air Corp ([[Bubble wrap|Bubble Wrap]], Polycap, Aircap)<br> |
Pregis (Astro-Cell; Astro-Bubble; Astro-Suprabubble)<br> | Pregis (Astro-Cell; Astro-Bubble; Astro-Suprabubble)<br> | ||
|- | |- | ||
| Line 40: | Line 40: | ||
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| − | PE; polietileno (Esp.); polythylne (Fr.); polietilene (It.); polietileno (Port.); polyolefin; polythene; alkathene | + | PE; HDPE; LDPE; polietileno (Esp.); polythylne (Fr.); polietilene (It.); polietileno (Port.); polyolefin; polythene; alkathene |
| − | Examples: [[Volara]]; [[Ethafoam]]; [[Tyvek]] [DuPont | + | Examples: [[Volara]]; [[Ethafoam]]; [[Tyvek]] [DuPont]; [[Tupperware]]; Lennite; Corrulite; Cellu-Cushion; Trirod; Ethalux; [[Plastazote]]; Colara; [[Correx]]; [[Polythene]]; Fortiflex; Poly-T; Sentinel; Cell-Aire, Microfoam |
| − | |||
== Applications == | == Applications == | ||
| − | * Storage containers | + | * Storage containers (storage and transport) |
| − | * Transparent sheets and films | + | * Transparent sheets and films (less expensive alternative to Mylar to protect from dust, abrasives and fingerprints) |
| − | * Foams | + | * Foams, rods and sheets (provides cushioning and support for storage and packaging; drawer liners) |
* Hot-melt adhesives | * Hot-melt adhesives | ||
| + | * Boards (used to create boxed and trays, backing for pictures and prints, shelf and drawer lining) | ||
* Spun-bonded fibers products | * Spun-bonded fibers products | ||
| + | |||
== Personal Risks == | == Personal Risks == | ||
Dow Corning: [http://hazard.com/msds/f2/bsc/bscjn.html SDS] | Dow Corning: [http://hazard.com/msds/f2/bsc/bscjn.html SDS] | ||
| Line 87: | Line 88: | ||
== Resources and Citations == | == Resources and Citations == | ||
<references/> | <references/> | ||
| + | * Omnexus: [https://omnexus.specialchem.com/selection-guide/polyethylene-plastic Guide on Polyethylenes] | ||
* Contributions: Gina Watkinson, AIC Plastics Panel, 2020. | * Contributions: Gina Watkinson, AIC Plastics Panel, 2020. | ||
* G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 304 | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 304 | ||
| − | |||
* Marjorie Shelley, ''The Care and Handling of Art Objects'', The Metropolitan Museum, New York, 1987 | * Marjorie Shelley, ''The Care and Handling of Art Objects'', The Metropolitan Museum, New York, 1987 | ||
| − | |||
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
| − | |||
* Rosalie Rosso King, ''Textile Identification, Conservation, and Preservation'', Noyes Publications, Park Ridge, NJ, 1985 | * Rosalie Rosso King, ''Textile Identification, Conservation, and Preservation'', Noyes Publications, Park Ridge, NJ, 1985 | ||
| − | |||
* Matt Roberts, Don Etherington, Matt Roberts, Don Etherington, ''Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology'', U.S. Government Printing Office, Washington DC, 1982 | * Matt Roberts, Don Etherington, Matt Roberts, Don Etherington, ''Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology'', U.S. Government Printing Office, Washington DC, 1982 | ||
| − | |||
* Pam Hatchfield, ''Pollutants in the Museum Environment'', Archetype Press, London, 2002 | * Pam Hatchfield, ''Pollutants in the Museum Environment'', Archetype Press, London, 2002 | ||
| − | |||
* Thomas C. Jester (ed.), ''Twentieth-Century Building Materials'', McGraw-Hill Companies, Washington DC, 1995 | * Thomas C. Jester (ed.), ''Twentieth-Century Building Materials'', McGraw-Hill Companies, Washington DC, 1995 | ||
| − | |||
* Random House, ''Webster's Encyclopedic Unabridged Dictionary of the English Language'', Grammercy Book, New York, 1997 | * Random House, ''Webster's Encyclopedic Unabridged Dictionary of the English Language'', Grammercy Book, New York, 1997 | ||
| − | |||
* M.Kaufman, ''The First Century of Plastics'', The Plastics and Rubber Institute, London, 1963 | * M.Kaufman, ''The First Century of Plastics'', The Plastics and Rubber Institute, London, 1963 | ||
| − | |||
* Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 | * Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 | ||
| − | |||
* Website address 1 Comment: www.nswpmith.com.au/historyofplastics.html | * Website address 1 Comment: www.nswpmith.com.au/historyofplastics.html | ||
| − | |||
* ''The Dictionary of Art'', Grove's Dictionaries Inc., New York, 1996 Comment: "Plastics" | * ''The Dictionary of Art'', Grove's Dictionaries Inc., New York, 1996 Comment: "Plastics" | ||
| − | |||
* Sharon Blank, An introduction to plastics and rubbers in collections, ''Studies in Conservation'', 35, 53-63, 1990 | * Sharon Blank, An introduction to plastics and rubbers in collections, ''Studies in Conservation'', 35, 53-63, 1990 | ||
| − | [[Category:Materials database]] | + | [[Category:Materials database]][[Category:MWG]][[Category: Sheet/Film, Plastic]] |
Latest revision as of 14:01, 2 October 2024
Description
A thermoplastic polymer in the polyolefin family with a base formula of [-CH2-]n. Ethylene was first polymerized in 1933 by ICI in England and was commercially released as Alkathene in 1939. In 1954, Karl Ziegler developed a process for high molecular weight polyethylene that allowed it to be spun into fibers, molded into durable but flexible forms and cast as tough thin sheets. Polyethylene made by the original process is now called Low density polyethylene (LDPE; density = 0.92, melting pt=110-120 C) because if has extensive branching resulting in a softer, more flexible product with low tensile strength. Polymers made by the later Ziegler or Phillips processes are called High density polyethylene or (HDPE; density=0.95-0.96, melting pt=130-138C) because they lave a low degree of branching resulting in high tensile strength. LDPE is softer, more flexible and has of lower tensile strength than HDPE [1], [2]. HDPE tends to have a longer lifespan than LDPE [1]. In general, polyethylenes are translucent waxy polymers with good impact strength that are widely used for packaging, coatings, liners, plastic sheets, wire coatings, underwater cables, containers, waste bags, toys, and squeeze bottles. Small amounts of additives (antioxidants, light stabilizers, slip agents, antistatic agents, flame retardants, pigments, etc.) are typically added to the final products. Polyethylene is recyclable and many products, such as Tyvek, contain the recycled polymer.
Examples include:
| Polyethylene Types | Forms | Products |
|---|---|---|
| High-Density Polyethylene (HDPE) | Closed cell foam
(non-crosslinked) |
Sealed Air Corp. formerly Dow; Ethafoam 180, 220, 400, 600, 900, 180 AS, 220 AS Pregis Corp; PolyPlank |
| High-Density Polyethylene (HDPE) | Closed-cell foam (cross-linked) | Sekisui Voltek; Volara
Zotefoams Ltd.; Plastazote MicroCell |
| High-Density Polyethylene (HDPE) | Spun bonded fiber | DuPont; Tyvek-10(stiff), Tyvek-14(soft), Tyvek-16(perforated) |
| Low-Density Polyethylene (LDPE) |
Closed cell foam (non-crosslinked) | Sealed Air Corp.(Cellu-Cushion; Celluplank; Cell-Aire; Stratocell |
| Low-Density Polyethylene (LDPE) | Plastic film with sealed bubbles | Sealed Air Corp (Bubble Wrap, Polycap, Aircap) Pregis (Astro-Cell; Astro-Bubble; Astro-Suprabubble) |
Some brands available in Antistatic (Pink), Flame-retardant (Blue), and Recycled (Green).
Synonyms and Related Terms
PE; HDPE; LDPE; polietileno (Esp.); polythylne (Fr.); polietilene (It.); polietileno (Port.); polyolefin; polythene; alkathene
Examples: Volara; Ethafoam; Tyvek [DuPont]; Tupperware; Lennite; Corrulite; Cellu-Cushion; Trirod; Ethalux; Plastazote; Colara; Correx; Polythene; Fortiflex; Poly-T; Sentinel; Cell-Aire, Microfoam
Applications
- Storage containers (storage and transport)
- Transparent sheets and films (less expensive alternative to Mylar to protect from dust, abrasives and fingerprints)
- Foams, rods and sheets (provides cushioning and support for storage and packaging; drawer liners)
- Hot-melt adhesives
- Boards (used to create boxed and trays, backing for pictures and prints, shelf and drawer lining)
- Spun-bonded fibers products
Personal Risks
Dow Corning: SDS
Collection Risks
- Degraded by ultraviolet light with discoloration, mechanical damage, and potential sulfur containing pollutants. Discoloration and mechanical damage will also occur in the dark at 50 degrees Celsius [2].
- May contain additives (such as antioxidant BHT) that can migrate to adjacent materials that may be absorbed by adjacent objects and cause staining [1].
- Slip agents such as alkyl amides may be transferred to objects
- Not as chemically stable as polyester films and not as clear.
- Polyethylene can be damaged by contact with objects that contain oils, fats, waxes or solvents., [1], [3]
- Slip agents, suck as alkyl amides may be transferred to objects
- Not susceptible to hydrolysis. “Highly hydrophobic polymers such as polyethylene and polypropylene are unlikely to have hydrolysable chemical groups, so are not subject to hydrolytic breakdown” [2].
Environmental Risks
Physical and Chemical Properties
- Soluble in xylene, trichlorobenzene, decane at room temperature and most chlorinated and aromatic solvents when gently heated.
- Insoluble in acetone, diethyl ether.
- Burns with yellow flame and blue center that smells like paraffin.
- Floats on water.
- CAS = 9002-88-4
- Melting Point = 110-138
- Density = 0.92-0.96
- Refractive Index = 1.52
Working Properties
Less transparent than polypropylene. Polyethylene has good chemical resistance.
Comparisons
Physical Properties for Selected Thermoplastic Resins
General Characteristics of Polymers
Resources and Citations
- ↑ 1.0 1.1 1.2 1.3 Tetreault, Jean. Products Used in Preventative Conservation- Technical Bulletin 32. https://www.canada.ca/en/conservation-institute/services/conservation-preservation-publications/technical-bulletins/products-used-preventive-conservation.html#a3b1e
- ↑ 2.0 2.1 2.2 Shashoua, Yvonne. Conservation of Plastics: Materials Science, Degradation and Preservation. Amsterdam etc.: Elsevier, 2008.
- ↑ Scott R. Williams. Plastic Storage Products. In ‘Preventive Conservation: Collection Storage’ Lisa Elkin and Christopher A. Norris (eds.), Society for the Preservation of Natural History Collections, New York. 2019. 773,774.
- Omnexus: Guide on Polyethylenes
- Contributions: Gina Watkinson, AIC Plastics Panel, 2020.
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 304
- Marjorie Shelley, The Care and Handling of Art Objects, The Metropolitan Museum, New York, 1987
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Rosalie Rosso King, Textile Identification, Conservation, and Preservation, Noyes Publications, Park Ridge, NJ, 1985
- Matt Roberts, Don Etherington, Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982
- Pam Hatchfield, Pollutants in the Museum Environment, Archetype Press, London, 2002
- Thomas C. Jester (ed.), Twentieth-Century Building Materials, McGraw-Hill Companies, Washington DC, 1995
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- M.Kaufman, The First Century of Plastics, The Plastics and Rubber Institute, London, 1963
- Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000
- Website address 1 Comment: www.nswpmith.com.au/historyofplastics.html
- The Dictionary of Art, Grove's Dictionaries Inc., New York, 1996 Comment: "Plastics"
- Sharon Blank, An introduction to plastics and rubbers in collections, Studies in Conservation, 35, 53-63, 1990