Difference between revisions of "Nylon 6,6"
| (22 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
| + | [[File:Nylon weave shoes.jpg|thumb|Nylon straw woven slippers<br>MFA# 1980.97]] | ||
[[File:nylon 6_6_blk.jpg|thumb|Nylon 6,6]] | [[File:nylon 6_6_blk.jpg|thumb|Nylon 6,6]] | ||
== Description == | == Description == | ||
| − | A polymer formed | + | A [[thermoplastic]] polymer formed by reacting a diamine with a diacid, often [[hexamethylenediamine]] with [[adipic acid]]. Nylon 6,6 was first developed in 1935 by W.H. Carothers at DuPont and patented in 1938 for use as a textile fiber. It was originally given the name Fiber #66. The fibers are known to be strong, tough, and elastic. Importantly, as opposed to traditional fibers such as cotton, wool, and rayon, nylon fibers are glossy.<ref>http://personal.strath.ac.uk/andrew.mclaren/Turin2002/CD%20congresso/The%20history%20of%20nylons.pdf</ref> They are extruded through a spinneret with a circular or trilobal cross section shape. Nylon 6,6 fibers can be used individually, i.e., as monofilaments, for brushes, surgical sutures, tennis strings, and fishing lines. Nylon 6,6 fibers are used for clothing, carpets, tire cords, conveyor belts, and brushes. Nylon 6,6 exhibits excellent dyeability and are twice as durable as [[cotton|cotton]] (see [http://Canvasetc.com/cotton_versus_nylon Canvasetc.com]). Nylon is sometimes coated with plastic, such as [[vinyl%20resin|vinyl]], to produce thin, lightweight waterproof fabrics. |
| + | The properties of nylon 6,6 are very similar to nylon 6, however the chemistry to synthesize these two polymers is different. | ||
See [[nylon%20fiber|nylon fiber]]. | See [[nylon%20fiber|nylon fiber]]. | ||
| + | Examples: Antron® [DuPont]; Cantrece® [DuPont]; Durasoft [Solutia]; Stainmaster [DuPont]; Wear-Dated [Solutia]; Brulon 240 [ICI]; Brulon 244 [ICI]; Perlon T [made in Germany] | ||
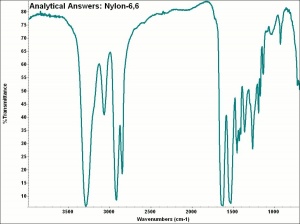
| + | [[[SliderGallery rightalign|aaiNYLON-66.jpg~FTIR]]] | ||
| + | == Synonyms and Related Terms == | ||
| + | |||
| + | polyamide 6,6; poly(hexamethylneadipamide); adipic nylon; Fiber #66; | ||
| − | + | == Personal Risks == | |
| − | == | + | |
| + | Nylon 6,6 is generally a safe material to handle. | ||
| + | |||
| + | American Polymer Standards Corporation: [http://www.ampolymer.com/SDS/Nylon6-6SDS.html SDS] | ||
| + | == Collection Risks == | ||
| − | + | * Can be sensitive to temperature fluctuations (Richardson et al 2014). | |
| + | * Typically stable although it was proposed to be a material ill-suited for use in conservation (Sease 1981). | ||
| + | * Nylon materials that contains plasticizers, additives or coatings should definitely be avoided (Tetreault 2017). | ||
| − | + | == Environmental Risks == | |
| − | + | Nylon 6,6 is not biodegradable. Manufacturing process releases nitrous oxide, a greenhouse gas. | |
| − | + | == Physical and Chemical Properties == | |
| − | + | Resistant to alkalis and most organic solvents. Degraded by acids and phenol. Burns with yellow-orange flame and blue smoke; smells of burnt horn. Fibers are smooth with no striations. | |
| − | + | * Cross section is circular or trilobal. | |
| − | + | * Tenacity = 4.6-9.0 g/denier (dry); 4.0-7.7 (wet); | |
| − | + | * Elongation = 19-40% (dry); 32-46% (wet); | |
| − | + | * Moisture regain = 3.8-4.5% (dry) | |
| − | + | * Melting Point = 250 | |
| − | + | * Density = 1.14 | |
| − | + | * Refractive Index = 1.521; 1.547 | |
| − | |||
| − | |||
| − | |||
== Comparisons == | == Comparisons == | ||
| Line 36: | Line 46: | ||
<gallery> | <gallery> | ||
| − | File:nylon 6_6_100X_pol.jpg|Nylon 6,6 | + | File:nylon 6_6_100X.jpg|thumb|Nylon 6,6 at 100x transmitted light |
| − | File:67 Nylon66 200X.jpg|Nylon 6,6 | + | File:nylon 6_6_100X_pol.jpg|Nylon 6,6 at 100x polarized light |
| − | File:67 Nylon66 200X pol.jpg|Nylon 6,6 | + | File:67 Nylon66 200X.jpg|Nylon 6,6 at 200x transmitted light |
| + | File:67 Nylon66 200X pol.jpg|Nylon 6,6 at 200x polarized light | ||
</gallery> | </gallery> | ||
| − | == | + | == Resources and Citations == |
| − | + | <references/> | |
| + | * Contributions: Catherine Stephens, AIC Plastics Panel, 2020. | ||
| + | * E. Richardson, G.Martin, P.Wyeth. (2014) Effects of heat on new and aged polyamide 6,6 textiles during pest eradication. Polymer degradation and stability 107 262-269. [https://www.sciencedirect.com/science/article/pii/S0141391013004138 link] | ||
| + | * Catherine Sease (1981) The Case against Using Soluble Nylon in Conservation Work Studies in Conservation Vol. 26 (3) 102-110; DOI: 10.2307/1505851 | ||
| + | * 'The History of Nylon' by Prof Trossarelli (Please scroll down if you don't initially see anything) [http://personal.strath.ac.uk/andrew.mclaren/Turin2002/CD%20congresso/The%20history%20of%20nylons.pdf pdf] | ||
| + | * Canvas etc.: https://www.canvasetc.com/cotton_versus_nylon/ (Accessed June 2020) | ||
| + | * Jean Tetreault ''Products Used in Preventive Conservation'' CCI, December 2017. [https://www.researchgate.net/publication/323153775_Products_Used_in_Preventive_Conservation Link] | ||
* G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 553 | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 553 | ||
| − | |||
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
| − | |||
* Marjory L. Joseph, ''Introductory Textile Science'', Holt, Rinehart and Winston, Fort Worth, TX, 1986, p. 135. | * Marjory L. Joseph, ''Introductory Textile Science'', Holt, Rinehart and Winston, Fort Worth, TX, 1986, p. 135. | ||
| − | |||
* J.Gordon Cook, ''Handbook of Textile Fibres:II Man-made Fibres'', Merrow Publishing Co., Durham, England | * J.Gordon Cook, ''Handbook of Textile Fibres:II Man-made Fibres'', Merrow Publishing Co., Durham, England | ||
| − | |||
* F. Kidd, ''Brushmaking Materials'', Bristish Brush Manufacturers, London, 1957 | * F. Kidd, ''Brushmaking Materials'', Bristish Brush Manufacturers, London, 1957 | ||
| − | |||
* Meredith Montague, contributed information, 1998 | * Meredith Montague, contributed information, 1998 | ||
| − | + | * History of Plastics: www.nswpmith.com.au/historyofplastics.html | |
| − | * | ||
| − | [[Category:Materials database]] | + | [[Category:Materials database]][[Category: MWG]][[Category: MWG]][[Category: Sheet/Film, Plastic]] |
Latest revision as of 14:05, 2 October 2024
Description
A Thermoplastic polymer formed by reacting a diamine with a diacid, often Hexamethylenediamine with Adipic acid. Nylon 6,6 was first developed in 1935 by W.H. Carothers at DuPont and patented in 1938 for use as a textile fiber. It was originally given the name Fiber #66. The fibers are known to be strong, tough, and elastic. Importantly, as opposed to traditional fibers such as cotton, wool, and rayon, nylon fibers are glossy.[1] They are extruded through a spinneret with a circular or trilobal cross section shape. Nylon 6,6 fibers can be used individually, i.e., as monofilaments, for brushes, surgical sutures, tennis strings, and fishing lines. Nylon 6,6 fibers are used for clothing, carpets, tire cords, conveyor belts, and brushes. Nylon 6,6 exhibits excellent dyeability and are twice as durable as Cotton (see Canvasetc.com). Nylon is sometimes coated with plastic, such as vinyl, to produce thin, lightweight waterproof fabrics.
The properties of nylon 6,6 are very similar to nylon 6, however the chemistry to synthesize these two polymers is different.
See Nylon fiber.
Examples: Antron® [DuPont]; Cantrece® [DuPont]; Durasoft [Solutia]; Stainmaster [DuPont]; Wear-Dated [Solutia]; Brulon 240 [ICI]; Brulon 244 [ICI]; Perlon T [made in Germany]
Synonyms and Related Terms
polyamide 6,6; poly(hexamethylneadipamide); adipic nylon; Fiber #66;
Personal Risks
Nylon 6,6 is generally a safe material to handle.
American Polymer Standards Corporation: SDS
Collection Risks
- Can be sensitive to temperature fluctuations (Richardson et al 2014).
- Typically stable although it was proposed to be a material ill-suited for use in conservation (Sease 1981).
- Nylon materials that contains plasticizers, additives or coatings should definitely be avoided (Tetreault 2017).
Environmental Risks
Nylon 6,6 is not biodegradable. Manufacturing process releases nitrous oxide, a greenhouse gas.
Physical and Chemical Properties
Resistant to alkalis and most organic solvents. Degraded by acids and phenol. Burns with yellow-orange flame and blue smoke; smells of burnt horn. Fibers are smooth with no striations.
- Cross section is circular or trilobal.
- Tenacity = 4.6-9.0 g/denier (dry); 4.0-7.7 (wet);
- Elongation = 19-40% (dry); 32-46% (wet);
- Moisture regain = 3.8-4.5% (dry)
- Melting Point = 250
- Density = 1.14
- Refractive Index = 1.521; 1.547
Comparisons
Properties of Synthetic Fibers
Additional Images
Resources and Citations
- Contributions: Catherine Stephens, AIC Plastics Panel, 2020.
- E. Richardson, G.Martin, P.Wyeth. (2014) Effects of heat on new and aged polyamide 6,6 textiles during pest eradication. Polymer degradation and stability 107 262-269. link
- Catherine Sease (1981) The Case against Using Soluble Nylon in Conservation Work Studies in Conservation Vol. 26 (3) 102-110; DOI: 10.2307/1505851
- 'The History of Nylon' by Prof Trossarelli (Please scroll down if you don't initially see anything) pdf
- Canvas etc.: https://www.canvasetc.com/cotton_versus_nylon/ (Accessed June 2020)
- Jean Tetreault Products Used in Preventive Conservation CCI, December 2017. Link
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 553
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Marjory L. Joseph, Introductory Textile Science, Holt, Rinehart and Winston, Fort Worth, TX, 1986, p. 135.
- J.Gordon Cook, Handbook of Textile Fibres:II Man-made Fibres, Merrow Publishing Co., Durham, England
- F. Kidd, Brushmaking Materials, Bristish Brush Manufacturers, London, 1957
- Meredith Montague, contributed information, 1998
- History of Plastics: www.nswpmith.com.au/historyofplastics.html