Difference between revisions of "Ammonium carbonate"
(username removed) |
|||
| (4 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | A white powder that smells strongly of [ | + | A white powder that smells strongly of [[ammonia%20%28anhydrous%29|ammonia]]. Ammonium carbonate is a double salt of [[ammonium%20carbamate|ammonium carbamate]] and [[ammonium%20bicarbonate|ammonium bicarbonate]]. Because it was historically prepared by the destructive distillation of the antlers from harts (red deer), ammonium carbonate is commonly called hartshorn. In air, it slowly decomposes to form ammonia and carbon dioxide. Ammonium carbonate is used in smelling salts, baking powder, fire extinguishers, ammonium casein glue, ceramics, and textile dyeing. It is also used as an aqueous neutralization/alkalization agent for paper (AIC Book and Paper catalog). Ammonium carbonate is also used in fire extinguishers, ceramics, photographic developing, washing wool, mordanting textiles, and coloring metals. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| − | hartshorn; spirit of hartshorn; volatile alkali; carbonate of ammonia; smelling salts; crystal ammonia; ammonium sesquicarbonate; sal volatile; rock ammonia; spiritus salis urinae | + | hartshorn; salt of hartshorn; spirit of hartshorn; volatile alkali; carbonate of ammonia; smelling salts; crystal ammonia; ammonium sesquicarbonate; sal volatile; rock ammonia; spiritus salis urinae |
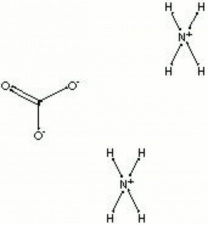
[[[SliderGallery rightalign|ammonium carbonate.jpg~Chemical structure]]] | [[[SliderGallery rightalign|ammonium carbonate.jpg~Chemical structure]]] | ||
| − | == | + | ==Risks== |
| + | * Noncombustible. | ||
| + | * Unstable in air evolving ammonia and carbon dioxide. | ||
| + | * Inhalation and skin contact causes irritation. | ||
| + | * Harmful if swallowed. | ||
| + | * Fisher Scientific: [https://fscimage.fishersci.com/msds/01160.htm#:~:text=MSDS%20Name%3A%20Ammonium%20carbonate%20Catalog%20Numbers%3A%20AC196650010%2C%20AC196651000%2C,A657-12%2C%20A657-212%2C%20A657-500%2C%20A657SAM1%2C%20A657SAM2%2C%20A657SAM3%2C%20BP2414-3%2C%20BP2414-500 SDS] | ||
| + | |||
| + | == Physical and Chemical Properties == | ||
Soluble in water. Partially soluble in ethanol. | Soluble in water. Partially soluble in ethanol. | ||
| Line 28: | Line 35: | ||
|- | |- | ||
! scope="row"| Boiling Point | ! scope="row"| Boiling Point | ||
| − | | 60 | + | | 60 |
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | * | + | * R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 |
| − | * | + | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 57 |
| − | * | + | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 |
| − | * | + | * Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, ''Technology and Conservation'', Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985 |
| − | * | + | * Book and Paper Group, ''Paper Conservation Catalog'', AIC, 1984, 1989 |
| − | * | + | * Random House, ''Webster's Encyclopedic Unabridged Dictionary of the English Language'', Grammercy Book, New York, 1997 |
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 534 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 534 | ||
| − | * | + | * External source or communication Comment: Submitted information from Budga Goran |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 11:21, 28 September 2022
Description
A white powder that smells strongly of ammonia. Ammonium carbonate is a double salt of Ammonium carbamate and Ammonium bicarbonate. Because it was historically prepared by the destructive distillation of the antlers from harts (red deer), ammonium carbonate is commonly called hartshorn. In air, it slowly decomposes to form ammonia and carbon dioxide. Ammonium carbonate is used in smelling salts, baking powder, fire extinguishers, ammonium casein glue, ceramics, and textile dyeing. It is also used as an aqueous neutralization/alkalization agent for paper (AIC Book and Paper catalog). Ammonium carbonate is also used in fire extinguishers, ceramics, photographic developing, washing wool, mordanting textiles, and coloring metals.
Synonyms and Related Terms
hartshorn; salt of hartshorn; spirit of hartshorn; volatile alkali; carbonate of ammonia; smelling salts; crystal ammonia; ammonium sesquicarbonate; sal volatile; rock ammonia; spiritus salis urinae
Risks
- Noncombustible.
- Unstable in air evolving ammonia and carbon dioxide.
- Inhalation and skin contact causes irritation.
- Harmful if swallowed.
- Fisher Scientific: SDS
Physical and Chemical Properties
Soluble in water. Partially soluble in ethanol.
| Composition | (NH4)HCO3-(NH4)CO2NH2 |
|---|---|
| CAS | 506-87-6 |
| Melting Point | 58 |
| Molecular Weight | mol. wt. = 96.09 |
| Boiling Point | 60 |
Resources and Citations
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 57
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985
- Book and Paper Group, Paper Conservation Catalog, AIC, 1984, 1989
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 534
- External source or communication Comment: Submitted information from Budga Goran