Difference between revisions of "Epsomite"
Jump to navigation
Jump to search
(username removed) |
|||
| (5 intermediate revisions by 4 users not shown) | |||
| Line 2: | Line 2: | ||
== Description == | == Description == | ||
| − | A [ | + | A [[magnesium sulfate]] mineral commonly found as an [[efflorescence]] on mine and cave walls. Epsomite was discovered at Epsom (Surrey England) in 1695 where it formed from the evaporation of mineral waters. The colorless or white salt often contains trace minerals, such as [[iron]] or [[calcium]]. Epsomite is purified and sold as Epsom salts for mineral baths. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 8: | Line 8: | ||
Epsom salts; bitter salts; | Epsom salts; bitter salts; | ||
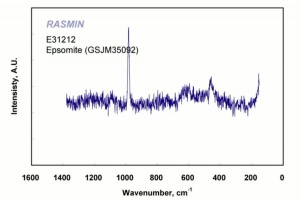
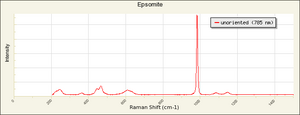
| − | [[[SliderGallery rightalign|epsomiteRS.jpg~Raman]]] | + | [[[SliderGallery rightalign|epsomiteRS.jpg~Raman (RASMIN)|Epsomite Raman RRUFF X050074.png~Raman (Caltech)]]] |
| + | == Physical and Chemical Properties == | ||
| − | + | * Soluble in water. | |
| − | + | * Orthorhombic crystals. | |
| − | Soluble in water. Orthorhombic crystals. Bitter to salty taste. | + | * Bitter to salty taste. |
| − | + | * Fracture = conchoidal. | |
| − | Fracture = conchoidal. Luster = vitreous to earthy. Streak = white | + | * Luster = vitreous to earthy. |
| + | * Streak = white | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 25: | Line 27: | ||
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 1.68 | + | | 1.68 g/ml |
|} | |} | ||
| − | == | + | == Resources and Citations == |
| − | |||
| − | |||
| − | + | * Mineralogy Database: [http://www.webmineral.com/data/Epsomite.shtml Epsomite] | |
| − | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: "epsomite" | + | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: "epsomite" [Accessed December 4, 2001](B/W photo) |
| − | * Wikipedia | + | * Wikipedia: http://en.wikipedia.org/wiki/Epsomite (Accessed Sept. 7, 2005) |
* ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | * ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | ||
| − | * | + | * Random House, ''Webster's Encyclopedic Unabridged Dictionary of the English Language'', Grammercy Book, New York, 1997 |
* ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | * ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | ||
Latest revision as of 12:12, 6 December 2022
Description
A Magnesium sulfate mineral commonly found as an Efflorescence on mine and cave walls. Epsomite was discovered at Epsom (Surrey England) in 1695 where it formed from the evaporation of mineral waters. The colorless or white salt often contains trace minerals, such as Iron or Calcium. Epsomite is purified and sold as Epsom salts for mineral baths.
Synonyms and Related Terms
Epsom salts; bitter salts;
Physical and Chemical Properties
- Soluble in water.
- Orthorhombic crystals.
- Bitter to salty taste.
- Fracture = conchoidal.
- Luster = vitreous to earthy.
- Streak = white
| Composition | MgSO4 - 7H2O |
|---|---|
| Mohs Hardness | 2.0 - 2.5 |
| Density | 1.68 g/ml |
Resources and Citations
- Mineralogy Database: Epsomite
- Encyclopedia Britannica, http://www.britannica.com Comment: "epsomite" [Accessed December 4, 2001](B/W photo)
- Wikipedia: http://en.wikipedia.org/wiki/Epsomite (Accessed Sept. 7, 2005)
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998