Difference between revisions of "Glycin"
Jump to navigation
Jump to search
(username removed) |
|||
| (3 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | A white, crystalline powder used as a black and white photographic developer. Glycin is as used as a colorimetric detection of [ | + | A white, crystalline powder used as a black and white photographic developer. Glycin is as used as a colorimetric detection of [[iron]], [[phosphorus]], and [[silicon]]. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
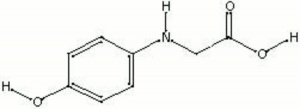
| + | [[[SliderGallery rightalign|glycin.jpg~Chemical structure]]] | ||
| + | p-hydroxyphenol aminoacetic acid; n-(4-hydroxyphenyl) glycine; photoglycine; glycine (photographic); Monazol; Ionyl | ||
| − | + | == Risks == | |
| − | [ | + | * Skin contact may cause irritation and allergies. |
| + | * Inhalation or ingestion may cause anemia, cyanosis, nausea, dizziness or difficulties in breathing. | ||
| + | * Fisher Scientific: [https://fscimage.fishersci.com/msds/83295.htm MSDS] | ||
| − | == | + | == Physical and Chemical Properties == |
Soluble in alkalis and acids. | Soluble in alkalis and acids. | ||
| − | Slightly soluble in water, | + | Slightly soluble in water, alcohol, acetone, ether, chloroform. |
{| class="wikitable" | {| class="wikitable" | ||
| Line 24: | Line 28: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 240 (dec) | + | | 240 C (dec) |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 30: | Line 34: | ||
|} | |} | ||
| − | == | + | == Resources and Citations == |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 4885 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 4885 | ||
| − | * | + | * Michael McCann, ''Artist Beware'', Watson-Guptill Publications, New York City, 1979 |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 07:57, 30 August 2022
Description
A white, crystalline powder used as a black and white photographic developer. Glycin is as used as a colorimetric detection of Iron, Phosphorus, and Silicon.
Synonyms and Related Terms
p-hydroxyphenol aminoacetic acid; n-(4-hydroxyphenyl) glycine; photoglycine; glycine (photographic); Monazol; Ionyl
Risks
- Skin contact may cause irritation and allergies.
- Inhalation or ingestion may cause anemia, cyanosis, nausea, dizziness or difficulties in breathing.
- Fisher Scientific: MSDS
Physical and Chemical Properties
Soluble in alkalis and acids.
Slightly soluble in water, alcohol, acetone, ether, chloroform.
| Composition | C8H9NO3 |
|---|---|
| CAS | 122-87-2 |
| Melting Point | 240 C (dec) |
| Molecular Weight | mol. wt. = 167.16 |
Resources and Citations
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 4885
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979