Difference between revisions of "Nickel acetate"
Jump to navigation
Jump to search
(username removed) |
|||
| (4 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | A green crystalline solid that is used as a [ | + | A green crystalline solid that is used as a [[mordant|mordant]] in textile dyeing. Nickel acetate is also used in [[nickel|nickel]] plating. |
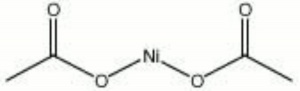
| − | + | [[[SliderGallery rightalign|nickel acetate.jpg~Chemical structure]]] | |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
nickel (II) acetate tetrahydrate | nickel (II) acetate tetrahydrate | ||
| − | [ | + | == Risks == |
| + | |||
| + | * Toxic by ingestion and inhalation. | ||
| + | * Skin contact can cause allergies. Carcinogen. | ||
| + | * Fisher Scientific: [https://fscimage.fishersci.com/msds/96308.htm MSDS] | ||
| − | == | + | ==Physical and Chemical Properties== |
Soluble in water, ethanol. | Soluble in water, ethanol. | ||
| Line 22: | Line 26: | ||
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 1.744 | + | | 1.744 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 28: | Line 32: | ||
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | * | + | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 |
| − | * | + | * Random House, ''Webster's Encyclopedic Unabridged Dictionary of the English Language'', Grammercy Book, New York, 1997 |
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 6583 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 6583 | ||
Latest revision as of 13:21, 23 August 2022
Description
A green crystalline solid that is used as a Mordant in textile dyeing. Nickel acetate is also used in Nickel plating.
Synonyms and Related Terms
nickel (II) acetate tetrahydrate
Risks
- Toxic by ingestion and inhalation.
- Skin contact can cause allergies. Carcinogen.
- Fisher Scientific: MSDS
Physical and Chemical Properties
Soluble in water, ethanol.
| Composition | Ni(OOCCH3)2-4H2O |
|---|---|
| CAS | 6018-89-9 |
| Density | 1.744 g/ml |
| Molecular Weight | mol. wt. = 248.78 |
Resources and Citations
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 6583