Difference between revisions of "Paraformaldehyde"
Jump to navigation
Jump to search
(username removed) |
|||
| (3 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | An amorphous white polymer of [ | + | An amorphous white polymer of [[formaldehyde|formaldehyde]]. Paraformaldehyde is used as a [[disinfectant|disinfectant]], [[fumigant|fumigant]], [[fungicide|fungicide]], and [[hardener|hardener]] for [[gelatin|gelatin]]. Because paraformaldehyde continually evolves gaseous formaldehyde, it is also used experimentally as a controlled source for formaldehyde exposure. |
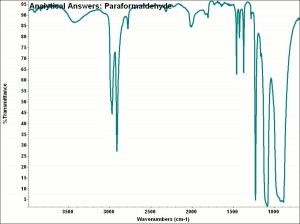
| − | + | [[[SliderGallery rightalign|aaiFORMALDHYDE.jpg~FTIR]]] | |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
polyoxymethylene; metaformaldehyde; polyformaldehyde; paraform; formagene; paraffins; Stopmildew (Durban, S.Africa) | polyoxymethylene; metaformaldehyde; polyformaldehyde; paraform; formagene; paraffins; Stopmildew (Durban, S.Africa) | ||
| − | [ | + | == Risks == |
| + | |||
| + | * Combustible. | ||
| + | * Flash point = 70C (158F). | ||
| + | * Toxic by ingestion and inhalation of vapors. | ||
| + | * Paraformaldehyde continually evolves formaldehyde fumes. | ||
| + | * CDH: [https://www.cdhfinechemical.com/images/product/msds/10_352091870_PARAFORMALDEHYDECASNO30525-89-4MSDS.pdf SDS] | ||
| − | == | + | == Physical and Chemical Properties == |
Soluble in strong alkalis. Insoluble in ethanol and ether. | Soluble in strong alkalis. Insoluble in ethanol and ether. | ||
| Line 22: | Line 28: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 120-170 | + | | 120-170 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 1.45 | + | | 1.45 g/ml |
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | * | + | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 342 |
| − | * | + | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 |
| − | * | + | * S.R.Trotman, E.R. Trotman, ''Textile Analysis'', J.B. Lippincott Company, Philadelphia, 1932 |
| − | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: "formaldehyde" | + | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: "formaldehyde" [Accessed 25 Jan. 2004]. |
| − | * Wikipedia | + | * Wikipedia: http://en.wikipedia.org/wiki/Paraformaldehyde (Accessed Feb. 10, 2006) |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 10:05, 26 July 2022
Description
An amorphous white polymer of Formaldehyde. Paraformaldehyde is used as a Disinfectant, Fumigant, Fungicide, and Hardener for Gelatin. Because paraformaldehyde continually evolves gaseous formaldehyde, it is also used experimentally as a controlled source for formaldehyde exposure.
Synonyms and Related Terms
polyoxymethylene; metaformaldehyde; polyformaldehyde; paraform; formagene; paraffins; Stopmildew (Durban, S.Africa)
Risks
- Combustible.
- Flash point = 70C (158F).
- Toxic by ingestion and inhalation of vapors.
- Paraformaldehyde continually evolves formaldehyde fumes.
- CDH: SDS
Physical and Chemical Properties
Soluble in strong alkalis. Insoluble in ethanol and ether.
| Composition | (HCHO)x |
|---|---|
| CAS | 30525-89-4 |
| Melting Point | 120-170 C |
| Density | 1.45 g/ml |
Resources and Citations
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 342
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- S.R.Trotman, E.R. Trotman, Textile Analysis, J.B. Lippincott Company, Philadelphia, 1932
- Encyclopedia Britannica, http://www.britannica.com Comment: "formaldehyde" [Accessed 25 Jan. 2004].
- Wikipedia: http://en.wikipedia.org/wiki/Paraformaldehyde (Accessed Feb. 10, 2006)