Difference between revisions of "Potassium nitrate"
Jump to navigation
Jump to search
(username removed) |
|||
| (4 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
[[File:pn31241nitre.jpg|thumb|Nitre]] | [[File:pn31241nitre.jpg|thumb|Nitre]] | ||
== Description == | == Description == | ||
| + | [[File:Potassium_nitratekes.jpg|thumb|Powdered possium nitrate]] | ||
| + | Clear colorless crystals that are slightly [[hygroscopic|hygroscopic]]. Potassium nitrate has been used since the 1300s as an ingredient in [[gunpowder|gunpowder]]. It was used in [[gold|gold]] smelting, [[glass|glass]] making, and [[textile|textile]] dyeing. In a closed environment, a [[saturated%20salt%20solutions|saturated salt solution]] of potassium nitrate will form an equilibrium at a relative humidity of about 93% (20C). | ||
| − | |||
| − | |||
| − | |||
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 10: | Line 9: | ||
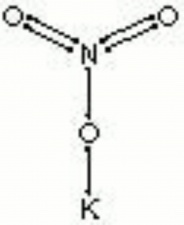
[[[SliderGallery rightalign|nitreRS.jpg~Raman|potassium nitrate.jpg~Chemical structure]]] | [[[SliderGallery rightalign|nitreRS.jpg~Raman|potassium nitrate.jpg~Chemical structure]]] | ||
| + | == Risks == | ||
| − | == | + | * Dangerous fire and explosion risk when shocked, heated or in contact with organic materials. |
| + | * Strong oxidizing agent. | ||
| + | * ThermoFisher: [https://www.fishersci.com/store/msds?partNumber=AC424155000&productDescription=POTASSIUM+NITRATE+REAGENT+500G&vendorId=VN00032119&countryCode=US&language=en SDS] | ||
| + | == Physical and Chemical Properties == | ||
Soluble in water, glycerol. Slightly soluble in ethanol. | Soluble in water, glycerol. Slightly soluble in ethanol. | ||
| − | Deliquescent point at 20C is 93.2 % RH (see [ | + | Deliquescent point at 20C is 93.2 % RH (see [[saturated%20salt%20solutions|saturated salt solutions]]) |
{| class="wikitable" | {| class="wikitable" | ||
| Line 26: | Line 29: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 333 | + | | 333 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 2.1062 | + | | 2.1062 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 35: | Line 38: | ||
|- | |- | ||
! scope="row"| Boiling Point | ! scope="row"| Boiling Point | ||
| − | | 400 (dec) | + | | 400 C (dec) |
|} | |} | ||
| − | == | + | == Resources and Citations == |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 632 | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 632 | ||
| Line 54: | Line 51: | ||
* Michael McCann, ''Artist Beware'', Watson-Guptill Publications, New York City, 1979 | * Michael McCann, ''Artist Beware'', Watson-Guptill Publications, New York City, 1979 | ||
| − | * Wikipedia | + | * Wikipedia: http://en.wikipedia.org/wiki/Potassium_nitrate (Accessed Nov. 9, 2005) |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 10:22, 25 August 2022
Description
Clear colorless crystals that are slightly Hygroscopic. Potassium nitrate has been used since the 1300s as an ingredient in Gunpowder. It was used in Gold smelting, Glass making, and Textile dyeing. In a closed environment, a saturated salt solution of potassium nitrate will form an equilibrium at a relative humidity of about 93% (20C).
Synonyms and Related Terms
niter; nitre; saltpeter; Bengal saltpeter ; sal prunella; kaliumnitrat (Dan., Deut.); salpêtre (Fr.); nitrato di potassio (It.); kaliumnitraat (Ned.); azotan potasu (Pol.); saletra potasowa (Pol.);
Risks
- Dangerous fire and explosion risk when shocked, heated or in contact with organic materials.
- Strong oxidizing agent.
- ThermoFisher: SDS
Physical and Chemical Properties
Soluble in water, glycerol. Slightly soluble in ethanol.
Deliquescent point at 20C is 93.2 % RH (see Saturated salt solutions)
| Composition | KNO3 |
|---|---|
| CAS | 7757-79-1 |
| Melting Point | 333 C |
| Density | 2.1062 g/ml |
| Molecular Weight | mol. wt. = 101.1 |
| Boiling Point | 400 C (dec) |
Resources and Citations
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 632
- Hermann Kuhn, Conservation and Restoration of Works of Art and Antiquities, Butterworths, London, 1986
- Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Wikipedia: http://en.wikipedia.org/wiki/Potassium_nitrate (Accessed Nov. 9, 2005)