Difference between revisions of "Aniline"
Jump to navigation
Jump to search
(username removed) |
|||
| (4 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
| + | [[File:MFA2018243 Aniline.jpg|thumb|Navajo child's blanket<br>MFA# 2018.243]] | ||
== Description == | == Description == | ||
| − | A colorless, oily liquid with a sweet amine-like odor. Aniline was first isolated in 1826 by Unverdorben and made synthetically in 1841 by Fritzsche. It was the base material for many of the early synthetic dyes. As a result, colors produces from coal-tar derivatives are generally called [ | + | A colorless, oily liquid with a sweet amine-like odor. Aniline was first isolated in 1826 by Unverdorben and made synthetically in 1841 by Fritzsche. It was the base material for many of the early synthetic dyes. As a result, colors produces from coal-tar derivatives are generally called [[aniline%20dye|aniline dyes]]. Aniline darkens to brown on exposure to air and light. Besides the manufacture of dyes, aniline is used as an [[accelerator|accelerator]] and [[antioxidant|antioxidant]] in rubbers. It is also used to make [[polyurethane|polyurethane]] foams, [[fungicide|fungicides]], and explosives. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 9: | Line 10: | ||
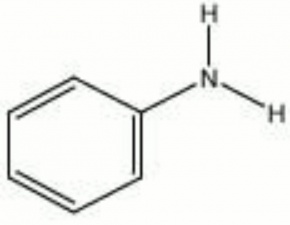
[[[SliderGallery rightalign|aniline.jpg~Chemical structure]]] | [[[SliderGallery rightalign|aniline.jpg~Chemical structure]]] | ||
| − | == | + | == Risks == |
| + | |||
| + | * Highly toxic by ingestion, inhalation and skin absorption. | ||
| + | * Combustible forming toxic nitrogen oxides and aniline vapor. | ||
| + | * Fisher Scientific: [https://beta-static.fishersci.com/content/dam/fishersci/en_US/documents/programs/education/regulatory-documents/sds/chemicals/chemicals-a/S25179.pdf SDS] | ||
| + | |||
| + | == Physical and Chemical Properties == | ||
Soluble in ethanol, ether and benzene. Slightly soluble in water. | Soluble in ethanol, ether and benzene. Slightly soluble in water. | ||
| Line 22: | Line 29: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | -6.2 | + | | -6.2 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 1.022 | + | | 1.022 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 34: | Line 41: | ||
|- | |- | ||
! scope="row"| Boiling Point | ! scope="row"| Boiling Point | ||
| − | | 184-186 | + | | 184-186 C |
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 59 | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 59 | ||
| Line 59: | Line 60: | ||
* Theodore J. Reinhart, 'Glossary of Terms', ''Engineered Plastics'', ASM International, 1988 Comment: [http:/www.ekornes.com/stressless/leaglos1.html/ Link] | * Theodore J. Reinhart, 'Glossary of Terms', ''Engineered Plastics'', ASM International, 1988 Comment: [http:/www.ekornes.com/stressless/leaglos1.html/ Link] | ||
| − | * | + | * Laboratory Safety Information: [http://www.qrc.com/hhmi/science/labsafe/lcss/lcss12.htm Link ] |
* ''CRC Handbook of Chemistry and Physics'', Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: ref. index=1.583 | * ''CRC Handbook of Chemistry and Physics'', Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: ref. index=1.583 | ||
Latest revision as of 09:41, 24 April 2022
Description
A colorless, oily liquid with a sweet amine-like odor. Aniline was first isolated in 1826 by Unverdorben and made synthetically in 1841 by Fritzsche. It was the base material for many of the early synthetic dyes. As a result, colors produces from coal-tar derivatives are generally called aniline dyes. Aniline darkens to brown on exposure to air and light. Besides the manufacture of dyes, aniline is used as an Accelerator and Antioxidant in rubbers. It is also used to make Polyurethane foams, fungicides, and explosives.
Synonyms and Related Terms
aminobenzene; benzenamine; phenylamine; aminophen; aniline oil; krstallin; kyanol
Risks
- Highly toxic by ingestion, inhalation and skin absorption.
- Combustible forming toxic nitrogen oxides and aniline vapor.
- Fisher Scientific: SDS
Physical and Chemical Properties
Soluble in ethanol, ether and benzene. Slightly soluble in water.
| Composition | C6H5NH2 |
|---|---|
| CAS | 62-53-3 |
| Melting Point | -6.2 C |
| Density | 1.022 g/ml |
| Molecular Weight | mol. wt. = 93.1 |
| Refractive Index | 1.583 |
| Boiling Point | 184-186 C |
Resources and Citations
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 59
- Encyclopedia Britannica, http://www.britannica.com Comment: "aniline" [Accessed May 22, 2003].
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983
- Theodore J. Reinhart, 'Glossary of Terms', Engineered Plastics, ASM International, 1988 Comment: [http:/www.ekornes.com/stressless/leaglos1.html/ Link]
- Laboratory Safety Information: Link
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: ref. index=1.583