Difference between revisions of "Glycerol"
(username removed) |
|||
| (6 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | A transparent, colorless, viscous liquid that is hygroscopic and has a sweet taste. Glycerol, first isolated in 1779 by Scheele, is a byproduct from the saponification of fats and oils. It is used in the production of alkyd resins, ester gums and dynamite. The thick, neutral liquid is also used as an emulsifier/plasticizer in printing inks, watercolor and gouache paints, glues, cements, and regenerated cellulose ([ | + | A transparent, colorless, viscous liquid that is hygroscopic and has a sweet taste. Glycerol, first isolated in 1779 by Scheele, is a byproduct from the saponification of fats and oils. It is used in the production of alkyd resins, ester gums and dynamite. The thick, neutral liquid is also used as an emulsifier/plasticizer in printing inks, watercolor and gouache paints, glues, cements, and regenerated cellulose ([[viscose rayon|rayon]], [[cellophane]]). In some formulations, glycerol is substituted with [[sorbitol]], or [[corn syrup]]. The term glycerin is used for commercial materials containing more than 95 percent glycerol. |
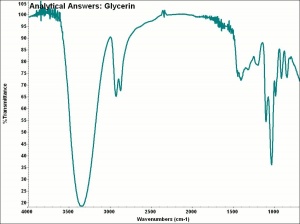
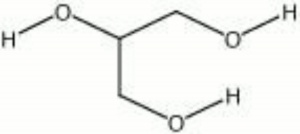
| − | + | [[[SliderGallery rightalign|aaiGLYCERIN.jpg~FTIR|glycerol.jpg~Chemical structure]]] | |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
glycerin; glycerine; glycol alcohol; 1,2,3-propanetriol; trihydroxypropane; sweet oil; glyceryl alcohol | glycerin; glycerine; glycol alcohol; 1,2,3-propanetriol; trihydroxypropane; sweet oil; glyceryl alcohol | ||
| − | [ | + | == Risks == |
| + | |||
| + | * Combustible. May explode if mixed with strong oxidizing agents such as chromium trioxide, potassium chlorate or potassium permanganate. | ||
| + | * ThermoFisher: [https://www.fishersci.com/store/msds?partNumber=AA4388330&productDescription=GLYCEROL+MONOSTEARATE+PFD+250G&vendorId=VN00024248&countryCode=US&language=en SDS] | ||
| + | * Most references consider glycerol a green solvent because it is bio-based, relatively non-toxic and biodegradable; its drawback for use is viscosity. | ||
| − | == | + | == Physical and Chemical Properties == |
Miscible with water, ethanol. Insoluble in ether, benzene and chlorinated hydrocarbons. | Miscible with water, ethanol. Insoluble in ether, benzene and chlorinated hydrocarbons. | ||
| Line 22: | Line 26: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 17.8 | + | | 17.8 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 1.2653 | + | | 1.2653 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 31: | Line 35: | ||
|- | |- | ||
! scope="row"| Boiling Point | ! scope="row"| Boiling Point | ||
| − | | 290 | + | | 290 C |
|} | |} | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
== Comparisons == | == Comparisons == | ||
| − | [[media: | + | [[media:download_file_138.pdf|Properties of Common Solvents]] |
| − | |||
| − | |||
| − | == | + | == Resources and Citations == |
| − | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: Glycerol. Retrieved June 1, 2003 | + | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: Glycerol. Retrieved June 1, 2003. |
* R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 | * R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 | ||
| Line 60: | Line 56: | ||
* S.R.Trotman, E.R. Trotman, ''Textile Analysis'', J.B. Lippincott Company, Philadelphia, 1932 | * S.R.Trotman, E.R. Trotman, ''Textile Analysis'', J.B. Lippincott Company, Philadelphia, 1932 | ||
| − | * | + | * Conservation termlist : www.hants.org.uk/museums |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 12:34, 17 April 2024
Description
A transparent, colorless, viscous liquid that is hygroscopic and has a sweet taste. Glycerol, first isolated in 1779 by Scheele, is a byproduct from the saponification of fats and oils. It is used in the production of alkyd resins, ester gums and dynamite. The thick, neutral liquid is also used as an emulsifier/plasticizer in printing inks, watercolor and gouache paints, glues, cements, and regenerated cellulose (rayon, Cellophane). In some formulations, glycerol is substituted with Sorbitol, or Corn syrup. The term glycerin is used for commercial materials containing more than 95 percent glycerol.
Synonyms and Related Terms
glycerin; glycerine; glycol alcohol; 1,2,3-propanetriol; trihydroxypropane; sweet oil; glyceryl alcohol
Risks
- Combustible. May explode if mixed with strong oxidizing agents such as chromium trioxide, potassium chlorate or potassium permanganate.
- ThermoFisher: SDS
- Most references consider glycerol a green solvent because it is bio-based, relatively non-toxic and biodegradable; its drawback for use is viscosity.
Physical and Chemical Properties
Miscible with water, ethanol. Insoluble in ether, benzene and chlorinated hydrocarbons.
| Composition | C3H8O3 |
|---|---|
| CAS | 56-81-5 |
| Melting Point | 17.8 C |
| Density | 1.2653 g/ml |
| Molecular Weight | mol. wt. = 92.1 |
| Boiling Point | 290 C |
Comparisons
Resources and Citations
- Encyclopedia Britannica, http://www.britannica.com Comment: Glycerol. Retrieved June 1, 2003.
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982
- Reed Kay, The Painter's Guide To Studio Methods and Materials, Prentice-Hall, Inc., Englewood Cliffs, NJ, 1983
- S.R.Trotman, E.R. Trotman, Textile Analysis, J.B. Lippincott Company, Philadelphia, 1932
- Conservation termlist : www.hants.org.uk/museums