Difference between revisions of "Cetalkonium chloride"
Jump to navigation
Jump to search
(username removed) |
|||
| (2 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | An odorless antiseptic compound widely used as a [ | + | An odorless antiseptic compound widely used as a [[disinfectant]] in [[detergent|detergents]]. Cetol is active at low concentrations and has been used to kill bacteria, [[fungus|fungi]], [[algae]], and [[lichen|lichens]]. It does not kill spores. The compounds effectiveness is reduced in solutions containing hard water, salts, or organic compounds. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 9: | Line 9: | ||
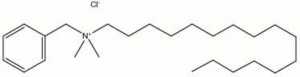
[[[SliderGallery rightalign|cetalkonium chloride.jpg~Chemical structure]]] | [[[SliderGallery rightalign|cetalkonium chloride.jpg~Chemical structure]]] | ||
| − | == | + | == Risks == |
| + | |||
| + | * Contact may cause skin irritation. | ||
| + | * An overdose may cause shortness of breath, cyanosis, CNS depression, low blood pressure, coma. | ||
| + | * Fisher Scientific: [https://fscimage.fishersci.com/msds/88331.htm MSDS] | ||
| + | |||
| + | ==Physical and Chemical Properties== | ||
Soluble in sorbitol solutions, glycerol, ether | Soluble in sorbitol solutions, glycerol, ether | ||
| Line 22: | Line 28: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 59 | + | | 59 C |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 28: | Line 34: | ||
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* G.Caneva, M.P.Nugari, O.Salvadori, ''Biology in the Conservation of Works of Art'', ICCROM, Rome, 1991 | * G.Caneva, M.P.Nugari, O.Salvadori, ''Biology in the Conservation of Works of Art'', ICCROM, Rome, 1991 | ||
| − | * | + | * e-DOC at http://www.edoc.co.za/medilink/actives/194.html |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 08:07, 28 May 2022
Description
An odorless antiseptic compound widely used as a Disinfectant in detergents. Cetol is active at low concentrations and has been used to kill bacteria, fungi, Algae, and lichens. It does not kill spores. The compounds effectiveness is reduced in solutions containing hard water, salts, or organic compounds.
Synonyms and Related Terms
benzyldimethylhexadecylammonium chloride; N-hexadecyl-N,N-dimethylbenzenemethanaminium chloride; Cetol; quaternary ammonium compounds
Risks
- Contact may cause skin irritation.
- An overdose may cause shortness of breath, cyanosis, CNS depression, low blood pressure, coma.
- Fisher Scientific: MSDS
Physical and Chemical Properties
Soluble in sorbitol solutions, glycerol, ether
| Composition | C25H46ClN.H2O |
|---|---|
| CAS | 122-18-9 |
| Melting Point | 59 C |
| Molecular Weight | mol. wt. = 395.78 |
Resources and Citations
- G.Caneva, M.P.Nugari, O.Salvadori, Biology in the Conservation of Works of Art, ICCROM, Rome, 1991