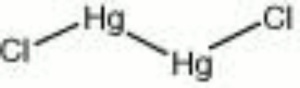Difference between revisions of "Mercurous chloride"
Jump to navigation
Jump to search
(username removed) |
|||
| (6 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | An odorless, white crystalline powder. Mercurous chloride, commonly known as calomel, has been used for many years as an [ | + | An odorless, white crystalline powder. Mercurous chloride, commonly known as calomel, has been used for many years as an [[insecticide|insecticide]], disinfectant, and [[desiccant|desiccant]]. It is used to make calomel paper, calomel electrodes, and as a [[fungicide|fungicide]]. Mercurous chloride is also used with [[gold|gold]] for [[amalgam%20gilding|mercury gilding]] on [[porcelain|porcelain]]. One Peruvian cabinet decorated with mopa-mopa, was found to contain calomel as a white pigment (Newman 2015). |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 9: | Line 9: | ||
[[[SliderGallery rightalign|mercurous chloride.jpg~Chemical structure]]] | [[[SliderGallery rightalign|mercurous chloride.jpg~Chemical structure]]] | ||
| − | == | + | == Risks == |
| − | Insoluble in water, ethanol, ether and cold dilute acids. Blackens with exposure to light, ammonia, and alkalis. | + | * Highly toxic by ingestion, inhalation and skin absorption. |
| + | * Echemi: [https://www.echemi.com/sds/calomel-pid_Rock20677.html SDS] | ||
| + | |||
| + | ==Physical and Chemical Properties== | ||
| + | |||
| + | * Insoluble in water, ethanol, ether and cold dilute acids. | ||
| + | * Blackens with exposure to light, ammonia, and alkalis. | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 22: | Line 28: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 302 | + | | 302 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 7.15 | + | | 7.15 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 31: | Line 37: | ||
|- | |- | ||
! scope="row"| Boiling Point | ! scope="row"| Boiling Point | ||
| − | | 384 | + | | 384 C |
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | + | * R. Newman, E. Kaplan, M. Derrick, “Mopa mopa: Scientific analysis and history of an unusual South American resin used by the Inka and artisans in Pasto, Colombia,” Journal of the American Institute for Conservation 54 (2015): 123-148. | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* ''The Merck Index'', Susan Budavari (ed.), Merck Research Labs, Whitehouse Station, NJ, 12th Edition, 1996 Comment: entry 5951 | * ''The Merck Index'', Susan Budavari (ed.), Merck Research Labs, Whitehouse Station, NJ, 12th Edition, 1996 Comment: entry 5951 | ||
Latest revision as of 12:45, 18 October 2022
Description
An odorless, white crystalline powder. Mercurous chloride, commonly known as calomel, has been used for many years as an Insecticide, disinfectant, and Desiccant. It is used to make calomel paper, calomel electrodes, and as a Fungicide. Mercurous chloride is also used with Gold for mercury gilding on Porcelain. One Peruvian cabinet decorated with mopa-mopa, was found to contain calomel as a white pigment (Newman 2015).
Synonyms and Related Terms
calomel; mild mercury chloride; mercury monochloride; mercury protochloride; mercury subchloride; precipite' blanc; Calogreen
Risks
- Highly toxic by ingestion, inhalation and skin absorption.
- Echemi: SDS
Physical and Chemical Properties
- Insoluble in water, ethanol, ether and cold dilute acids.
- Blackens with exposure to light, ammonia, and alkalis.
| Composition | Hg2Cl2 |
|---|---|
| CAS | 10112-91-1 |
| Melting Point | 302 C |
| Density | 7.15 g/ml |
| Molecular Weight | mol. wt. = 472.09 |
| Boiling Point | 384 C |
Resources and Citations
- R. Newman, E. Kaplan, M. Derrick, “Mopa mopa: Scientific analysis and history of an unusual South American resin used by the Inka and artisans in Pasto, Colombia,” Journal of the American Institute for Conservation 54 (2015): 123-148.
- The Merck Index, Susan Budavari (ed.), Merck Research Labs, Whitehouse Station, NJ, 12th Edition, 1996 Comment: entry 5951
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993