Difference between revisions of "Paratoluidine"
Jump to navigation
Jump to search
(username removed) |
|||
| (2 intermediate revisions by one other user not shown) | |||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | Transparent colorless crystals prepared by treating nitrotoluene with [ | + | Transparent colorless crystals prepared by treating nitrotoluene with [[acetic%20acid|acetic acid]] in the presence of [[iron|iron]]. Paratoluidine is used in the manufacture of some synthetic organic red colorants. Paratoluidine reds are sometimes used in inks but are not used as an artist colors because they bleed and fade with time. |
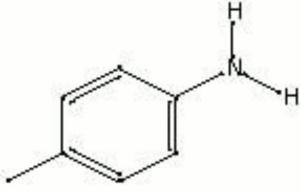
| − | + | [[[SliderGallery rightalign|paratoluidine.jpg~Chemical structure]]] | |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
p-toluidine; 4-aminotoluene; 4-amino-1-methylbenzene; p-methylaniline | p-toluidine; 4-aminotoluene; 4-amino-1-methylbenzene; p-methylaniline | ||
| − | [ | + | == Risks == |
| + | |||
| + | * Toxic by ingestion, inhalation and skin absorption. | ||
| + | * Combustible. Flash point = 86C (188F) | ||
| + | * NIH: [https://pubchem.ncbi.nlm.nih.gov/compound/p-Toluidine Compound summary] | ||
| − | == | + | == Physical and Chemical Properties == |
Soluble in alcohols, ether, acetone, carbon disulfide, oils, dilute acids. Slightly soluble in water. | Soluble in alcohols, ether, acetone, carbon disulfide, oils, dilute acids. Slightly soluble in water. | ||
| Line 24: | Line 28: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 44-45 | + | | 44-45 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 1.046 | + | | 1.046 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 33: | Line 37: | ||
|- | |- | ||
! scope="row"| Boiling Point | ! scope="row"| Boiling Point | ||
| − | | 200-201 | + | | 200-201 C |
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 9396 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 9396 | ||
| − | |||
| − | |||
* F. Crace-Calvert, ''Dyeing and Calico Printing'', Palmer & Howe, London, 1876 | * F. Crace-Calvert, ''Dyeing and Calico Printing'', Palmer & Howe, London, 1876 | ||
Latest revision as of 13:36, 17 October 2022
Description
Transparent colorless crystals prepared by treating nitrotoluene with Acetic acid in the presence of Iron. Paratoluidine is used in the manufacture of some synthetic organic red colorants. Paratoluidine reds are sometimes used in inks but are not used as an artist colors because they bleed and fade with time.
Synonyms and Related Terms
p-toluidine; 4-aminotoluene; 4-amino-1-methylbenzene; p-methylaniline
Risks
- Toxic by ingestion, inhalation and skin absorption.
- Combustible. Flash point = 86C (188F)
- NIH: Compound summary
Physical and Chemical Properties
Soluble in alcohols, ether, acetone, carbon disulfide, oils, dilute acids. Slightly soluble in water.
Darkens on exposure to air.
| Composition | C6H4CH3NH2 |
|---|---|
| CAS | 106-49-0 |
| Melting Point | 44-45 C |
| Density | 1.046 g/ml |
| Molecular Weight | mol. wt. = 107.2 |
| Boiling Point | 200-201 C |
Resources and Citations
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 9396
- F. Crace-Calvert, Dyeing and Calico Printing, Palmer & Howe, London, 1876