Difference between revisions of "Phenol formaldehyde resin"
(username removed) |
(→Risks) |
||
| (8 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | A thermosetting [ | + | A thermosetting [[amino%20resin|amino resin]] that is made by reacting [[phenol|phenol]] with [[formaldehyde|formaldehyde]]. Discovered in 1907 by Leo Baekeland and sold as [[Bakelite|Bakelite]] in 1909, phenol formaldehyde resin was the first true synthetic plastics. Phenolic resins can be made with either excess formaldehyde (resol) or with an excess of phenol (novolac). Resols are soluble in alcohol while novolacs are solid at room temperature. Both require crosslinking tod form a hard plastic that is brittle but has good resistance to water and biodegradation. In the early 20th century, phenol formaldehyde resins were used for dark color molded plastic products sometimes filled with [[cellulose|cellulose]], [[wood%20flour|wood flour]], or mineral powders. Resol phenolics are currently used for plywood, textile sizing, leather processing, paper strengthening, foams and chemical resistant coatings. Novolacs are used for fibers, adhesives, molded parts, circuit boards, and mechanical fittings. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| − | phenol-formaldehyde resin (AAT); phenolic resin; PF; novolac; resol; | + | phenol-formaldehyde resin (AAT); phenolic resin; PF; novolac; resol |
| + | Commercial products: Bakelite (before 1939); Durex; Durite; Indur; Resinox; Novolac; Remonal | ||
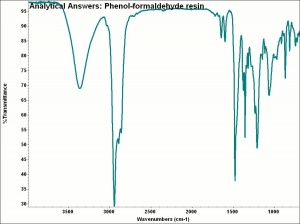
| + | [[[SliderGallery rightalign|aaiPHENYLFORMresin.jpg~FTIR]]] | ||
| + | ==Risks== | ||
| − | [ | + | * Evolves formaldehyde and ammonia as it degrades. |
| + | * Can corrode copper and brass. | ||
| + | * Cracking, discoloration, fading, blooming | ||
| + | * Plenco: [https://plenco.com/data/SDS/02567.PDF SDS] | ||
| − | == | + | == Physical and Chemical Properties == |
Sensitive to acids and alkalis | Sensitive to acids and alkalis | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
== Comparisons == | == Comparisons == | ||
| Line 31: | Line 25: | ||
[[media:download_file_281.pdf|Physical Properties for Selected Thermoset Resins]] | [[media:download_file_281.pdf|Physical Properties for Selected Thermoset Resins]] | ||
| − | + | == Resources and Citations == | |
| − | + | * [https://www.nps.gov/museum/publications/conserveogram/08-04.pdf Care and Identification of Objects Made from Plastic], Conserve O Gram 8/4, National Park Service, September 2010. | |
| − | == | ||
| − | |||
* G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 598 | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 598 | ||
| Line 45: | Line 37: | ||
* Sharon Blank, An introduction to plastics and rubbers in collections, ''Studies in Conservation'', 35, 53-63, 1990 | * Sharon Blank, An introduction to plastics and rubbers in collections, ''Studies in Conservation'', 35, 53-63, 1990 | ||
| − | * Art and Architecture Thesaurus Online, | + | * Art and Architecture Thesaurus Online, https://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 07:16, 22 October 2022
Description
A thermosetting Amino resin that is made by reacting Phenol with Formaldehyde. Discovered in 1907 by Leo Baekeland and sold as Bakelite in 1909, phenol formaldehyde resin was the first true synthetic plastics. Phenolic resins can be made with either excess formaldehyde (resol) or with an excess of phenol (novolac). Resols are soluble in alcohol while novolacs are solid at room temperature. Both require crosslinking tod form a hard plastic that is brittle but has good resistance to water and biodegradation. In the early 20th century, phenol formaldehyde resins were used for dark color molded plastic products sometimes filled with Cellulose, Wood flour, or mineral powders. Resol phenolics are currently used for plywood, textile sizing, leather processing, paper strengthening, foams and chemical resistant coatings. Novolacs are used for fibers, adhesives, molded parts, circuit boards, and mechanical fittings.
Synonyms and Related Terms
phenol-formaldehyde resin (AAT); phenolic resin; PF; novolac; resol
Commercial products: Bakelite (before 1939); Durex; Durite; Indur; Resinox; Novolac; Remonal
Risks
- Evolves formaldehyde and ammonia as it degrades.
- Can corrode copper and brass.
- Cracking, discoloration, fading, blooming
- Plenco: SDS
Physical and Chemical Properties
Sensitive to acids and alkalis
Comparisons
General Characteristics of Polymers
Physical Properties for Selected Thermoset Resins
Resources and Citations
- Care and Identification of Objects Made from Plastic, Conserve O Gram 8/4, National Park Service, September 2010.
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 598
- Dictionary of Building Preservation, Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996
- Pam Hatchfield, Pollutants in the Museum Environment, Archetype Press, London, 2002
- Thomas C. Jester (ed.), Twentieth-Century Building Materials, McGraw-Hill Companies, Washington DC, 1995
- Sharon Blank, An introduction to plastics and rubbers in collections, Studies in Conservation, 35, 53-63, 1990
- Art and Architecture Thesaurus Online, https://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000