Difference between revisions of "Antlerite"
Jump to navigation
Jump to search

(username removed) |
|||
| (9 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
| + | [[File:Antlerite R050212 Sample Photo 19588 M.jpg|thumb|Antlerite; creidt: [https://rruff.info/antlerite/display=default/R050212 RRUFF]]] | ||
== Description == | == Description == | ||
| − | An uncommon mineral composed of [ | + | An uncommon mineral composed of [[copper%20sulfate%2C%20dibasic|dibasic copper sulfate]]. Antlerite is an emerald green to blackish-green color and is found in the oxidized zones of copper deposits of Arizona and Chile. It has also been identified as a corrosion product on outdoor bronze and copper sculpture. Though similar in color to [[malachite|malachite]] and [[brochantite|brochantite]], antlerite does not effervesce in contact with acid. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 7: | Line 8: | ||
antlerita (Esp.); antierite (Port.); Antlerit (Deut.); antleriet (Ned.) | antlerita (Esp.); antierite (Port.); Antlerit (Deut.); antleriet (Ned.) | ||
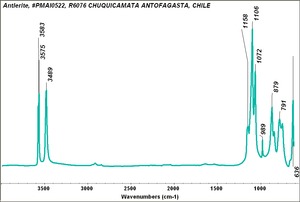
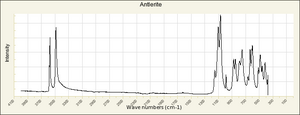
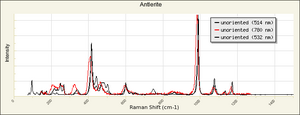
| − | [[[SliderGallery rightalign| | + | [[[SliderGallery rightalign|Antlerite PMA.TIF~FTIR (PMA)|Antlerite infrared RRUFF R050212.png~IR-ATR (RRUFF)|Antlerite Raman RRUFF R050212.png~Raman (RRUFF)]]] |
| − | |||
| − | |||
| + | == Physical and Chemical Properties == | ||
| + | An | ||
Crystal system = orthorhombic bipyramidal Cleavage = unidirectional Fracture = uneven Luster = vitreous Streak=pale green | Crystal system = orthorhombic bipyramidal Cleavage = unidirectional Fracture = uneven Luster = vitreous Streak=pale green | ||
| Line 22: | Line 23: | ||
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 3.88 | + | | 3.88 g/ml |
|- | |- | ||
! scope="row"| Refractive Index | ! scope="row"| Refractive Index | ||
| Line 28: | Line 29: | ||
|} | |} | ||
| − | == | + | == Resources and Citations == |
| + | * Mindat.org: [https://www.mindat.org/min-268.html antlerite] | ||
* ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | * ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | ||
| Line 34: | Line 36: | ||
* Random House, ''Webster's Encyclopedic Unabridged Dictionary of the English Language'', Grammercy Book, New York, 1997 | * Random House, ''Webster's Encyclopedic Unabridged Dictionary of the English Language'', Grammercy Book, New York, 1997 | ||
| − | * Wikipedia | + | * Wikipedia: http://en.wikipedia.org/wiki/Antlerite (Accessed Mar. 20, 2006) |
* Thomas Gregory, ''The Condensed Chemical Dictionary'', Reinhold Publishing, New York, 3rd ed., 1942 | * Thomas Gregory, ''The Condensed Chemical Dictionary'', Reinhold Publishing, New York, 3rd ed., 1942 | ||
Latest revision as of 10:46, 8 December 2022

Antlerite; creidt: RRUFF
Description
An uncommon mineral composed of dibasic copper sulfate. Antlerite is an emerald green to blackish-green color and is found in the oxidized zones of copper deposits of Arizona and Chile. It has also been identified as a corrosion product on outdoor bronze and copper sculpture. Though similar in color to Malachite and Brochantite, antlerite does not effervesce in contact with acid.
Synonyms and Related Terms
antlerita (Esp.); antierite (Port.); Antlerit (Deut.); antleriet (Ned.)
Physical and Chemical Properties
An Crystal system = orthorhombic bipyramidal Cleavage = unidirectional Fracture = uneven Luster = vitreous Streak=pale green
| Composition | Cu3(OH)4SO4 |
|---|---|
| Mohs Hardness | 3.5-4.0 |
| Density | 3.88 g/ml |
| Refractive Index | 1.72-1.78 |
Resources and Citations
- Mindat.org: antlerite
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- Wikipedia: http://en.wikipedia.org/wiki/Antlerite (Accessed Mar. 20, 2006)
- Thomas Gregory, The Condensed Chemical Dictionary, Reinhold Publishing, New York, 3rd ed., 1942
- Dictionary of Building Preservation, Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996