Difference between revisions of "Lithopone"
(username removed) |
|||
| (5 intermediate revisions by 4 users not shown) | |||
| Line 2: | Line 2: | ||
== Description == | == Description == | ||
| − | A dense, white, opaque pigment composed of a mixture of [ | + | A dense, white, opaque pigment composed of a mixture of [[zinc sulfide]] (30%) and [[barium sulfate]] (70%) with trace amounts of [[zinc oxide]]. Lithopone, first produced in 1874, was called Orr's white. The mixture of the two components is so intimate that it is hard to distinguish microscopically. Lithopone is an inert, transparent pigment which is often used as a filler or as a base for lake pigments. Lithopone was widely used in house paints in the first half of the 20th century. It was also used for some artist grounds, inks and as a filler in [[paper]], [[leather]], and [[linoleum]]. Now lithopone has mostly been replaced by [[titanium dioxide]]. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| − | |||
Pigment White 5; CI 77115; Deckweiss (Deut.); Lithopone (Deut.); litopón (Esp.); lithopone (Fr.); lithoponio (Gr.); litopone (It.); lithopoon (Ned.); litopone (Port.); Orr's white; oleum white; Griffiths zinc white; Sterling white; Albalith; Charlton white; Ponolith; Jersey Lily white; Sunotlith; Beckton white; Zincolith | Pigment White 5; CI 77115; Deckweiss (Deut.); Lithopone (Deut.); litopón (Esp.); lithopone (Fr.); lithoponio (Gr.); litopone (It.); lithopoon (Ned.); litopone (Port.); Orr's white; oleum white; Griffiths zinc white; Sterling white; Albalith; Charlton white; Ponolith; Jersey Lily white; Sunotlith; Beckton white; Zincolith | ||
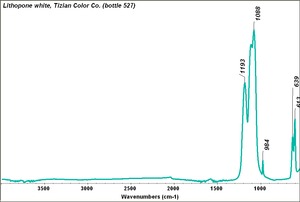
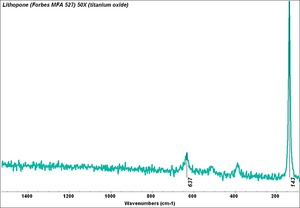
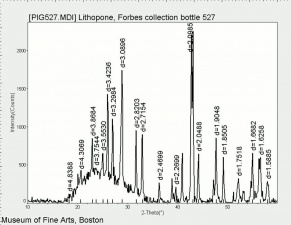
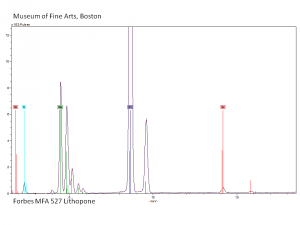
| + | [[[SliderGallery rightalign|Lithopone white, 527.TIF~FTIR (MFA)|Lithopone (Forbes MFA 527) 50X (titanium oxide) copy.tif~Raman (MFA)|PIG527.jpg~XRD|f527sem.jpg~SEM|f527edsbw.jpg~EDS|Slide20 F527.PNG~XRF]]] | ||
| − | + | == Risks == | |
| − | + | * Nonpoisonous. | |
| + | * Can darken in the presence of iron. | ||
| + | * May chalk with exposure to UV light. | ||
| − | + | == Physical and Chemical Properties == | |
| − | Soluble in HCl releasing sulfur fumes. | + | * Particle size = 0.3-0.5 micrometers |
| + | * Soluble in HCl releasing sulfur fumes. | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 22: | Line 25: | ||
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 4.3 | + | | 4.3 g/ml |
|- | |- | ||
! scope="row"| Refractive Index | ! scope="row"| Refractive Index | ||
| 2.3(ZnS), 1.64(BaSO4) | | 2.3(ZnS), 1.64(BaSO4) | ||
|} | |} | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
== Comparisons == | == Comparisons == | ||
| Line 38: | Line 35: | ||
[[media:download_file_516.pdf|Characteristics of Common White Pigments]] | [[media:download_file_516.pdf|Characteristics of Common White Pigments]] | ||
| − | + | ==Resources and Citations== | |
| − | |||
| − | == | ||
* Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, ''Pigment Compendium'', Elsevier Butterworth-Heinemann, Oxford, 2004 | * Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, ''Pigment Compendium'', Elsevier Butterworth-Heinemann, Oxford, 2004 | ||
Latest revision as of 15:53, 15 October 2022
Description
A dense, white, opaque pigment composed of a mixture of Zinc sulfide (30%) and Barium sulfate (70%) with trace amounts of Zinc oxide. Lithopone, first produced in 1874, was called Orr's white. The mixture of the two components is so intimate that it is hard to distinguish microscopically. Lithopone is an inert, transparent pigment which is often used as a filler or as a base for lake pigments. Lithopone was widely used in house paints in the first half of the 20th century. It was also used for some artist grounds, inks and as a filler in Paper, Leather, and Linoleum. Now lithopone has mostly been replaced by Titanium dioxide.
Synonyms and Related Terms
Pigment White 5; CI 77115; Deckweiss (Deut.); Lithopone (Deut.); litopón (Esp.); lithopone (Fr.); lithoponio (Gr.); litopone (It.); lithopoon (Ned.); litopone (Port.); Orr's white; oleum white; Griffiths zinc white; Sterling white; Albalith; Charlton white; Ponolith; Jersey Lily white; Sunotlith; Beckton white; Zincolith
Risks
- Nonpoisonous.
- Can darken in the presence of iron.
- May chalk with exposure to UV light.
Physical and Chemical Properties
- Particle size = 0.3-0.5 micrometers
- Soluble in HCl releasing sulfur fumes.
| CAS | 1345-05-7 |
|---|---|
| Density | 4.3 g/ml |
| Refractive Index | 2.3(ZnS), 1.64(BaSO4) |
Comparisons
Characteristics of Common White Pigments
Resources and Citations
- Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, Pigment Compendium, Elsevier Butterworth-Heinemann, Oxford, 2004
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966 Comment: density= 4.3 and ref.index.= 2.3 and 1.64
- Reed Kay, The Painter's Guide To Studio Methods and Materials, Prentice-Hall, Inc., Englewood Cliffs, NJ, 1983
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- The Dictionary of Art, Grove's Dictionaries Inc., New York, 1996 Comment: 'Pigments'
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 461
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982
- Dictionary of Building Preservation, Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- Thomas B. Brill, Light Its Interaction with Art and Antiquities, Plenum Press, New York City, 1980
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985
- Book and Paper Group, Paper Conservation Catalog, AIC, 1984, 1989
- Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000