Difference between revisions of "Erythrosine"
Jump to navigation
Jump to search
(username removed) |
|||
| (2 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
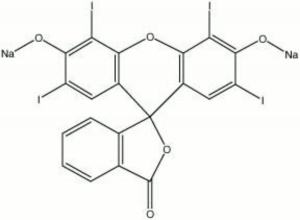
| − | A brown powder that forms a cherry red synthetic dye solution with water. First discovered in 1876 by Kussamaul, erythrosine is an iodinate derivative of [ | + | A brown powder that forms a cherry red synthetic dye solution with water. First discovered in 1876 by Kussamaul, erythrosine is an iodinate derivative of [[fluorescein]]. It is used as a direct dye on [[wool]] and [[silk]]. Erythrosine is also used in inks, lacquers, cosmetics, and as a lake pigment. It is not colorfast in [[solar radiation|sunlight]]. |
| + | [[[SliderGallery rightalign|erythrosine.jpg~Chemical structure]]] | ||
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
erythrosine B; erythrosine BS; Acid Red 51; CI 45430; FD&C Red No.3; Food Red 14; Pigment Red 172 (aluminum salt); Solvent Red 140; érythrosine (Fr.); eritrosina (Esp., Port.); sodium iodeosin; erythrosin | erythrosine B; erythrosine BS; Acid Red 51; CI 45430; FD&C Red No.3; Food Red 14; Pigment Red 172 (aluminum salt); Solvent Red 140; érythrosine (Fr.); eritrosina (Esp., Port.); sodium iodeosin; erythrosin | ||
| − | + | == Risks == | |
| − | |||
| − | == | ||
| − | + | * Toxicity and carcinogenicity are being studied. | |
| + | * MilliporeSigma: [https://www.emdmillipore.com/US/en/product/msds/MDA_CHEM-115936?ReferrerURL=https%3A%2F%2Fsearch.yahoo.com%2F&bd=1 SDS] | ||
| − | + | ==Physical and Chemical Properties== | |
| − | The fluorescence of erythrosine changes with pH. It is colorless at pH 4.0 changing to a | + | * Soluble in water, ethanol. |
| + | * An aqueous solution of erythrosine will form a yellow-brown precipitate when drops of HCl are added and a red precipitate when drops of NaOH solution are added. | ||
| + | * Maximum absorption wavelength = 524 nm. | ||
| + | * The fluorescence of erythrosine changes with pH. It is colorless at pH 4.0 changing to a fluorescent yellow-green at pH 4.5 | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 29: | Line 32: | ||
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | * M.Ballard (ed.), ''Important Early Synthetic Dyes. Chemistry, Constitution, Date, Properties.'' Conservation Analytical Laboratory, Smithsonian Institution, 1991. | |
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
| Line 45: | Line 40: | ||
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 3734 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 3734 | ||
| − | * | + | * T.Schafer, C. Norton, V.Blyth-Hill, "The Efficacy of Using Boards Containing Zeolites in Passepartout for Works of Art on Paper, WAAC Newsletter, 22(1):14, 2000. -erythrosine fades rapidly |
* Colour Index International online at www.colour-index.org Comment: discoverer = Kussmaul; CAS=16423-68-0, 12227-78-0, 15905-32-5 | * Colour Index International online at www.colour-index.org Comment: discoverer = Kussmaul; CAS=16423-68-0, 12227-78-0, 15905-32-5 | ||
Latest revision as of 14:16, 5 August 2022
Description
A brown powder that forms a cherry red synthetic dye solution with water. First discovered in 1876 by Kussamaul, erythrosine is an iodinate derivative of Fluorescein. It is used as a direct dye on Wool and Silk. Erythrosine is also used in inks, lacquers, cosmetics, and as a lake pigment. It is not colorfast in sunlight.
Synonyms and Related Terms
erythrosine B; erythrosine BS; Acid Red 51; CI 45430; FD&C Red No.3; Food Red 14; Pigment Red 172 (aluminum salt); Solvent Red 140; érythrosine (Fr.); eritrosina (Esp., Port.); sodium iodeosin; erythrosin
Risks
- Toxicity and carcinogenicity are being studied.
- MilliporeSigma: SDS
Physical and Chemical Properties
- Soluble in water, ethanol.
- An aqueous solution of erythrosine will form a yellow-brown precipitate when drops of HCl are added and a red precipitate when drops of NaOH solution are added.
- Maximum absorption wavelength = 524 nm.
- The fluorescence of erythrosine changes with pH. It is colorless at pH 4.0 changing to a fluorescent yellow-green at pH 4.5
| Composition | C20H6I4Na2O5 |
|---|---|
| CAS | 16423-68-0 |
| Molecular Weight | mol. wt. = 879.86 |
Resources and Citations
- M.Ballard (ed.), Important Early Synthetic Dyes. Chemistry, Constitution, Date, Properties. Conservation Analytical Laboratory, Smithsonian Institution, 1991.
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 3734
- T.Schafer, C. Norton, V.Blyth-Hill, "The Efficacy of Using Boards Containing Zeolites in Passepartout for Works of Art on Paper, WAAC Newsletter, 22(1):14, 2000. -erythrosine fades rapidly
- Colour Index International online at www.colour-index.org Comment: discoverer = Kussmaul; CAS=16423-68-0, 12227-78-0, 15905-32-5
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: The fluorescence of erythrosine changes with pH. It is colorless at pH 4.0 changing to a fluorescescent yellow-green at pH 4.5
- Sigma Dyes, Stains and Natural Pigments, Infrared Library, Nicolet, 1991-1995 Comment: OMNIC for Erythrosine B: formula= C20H8I4O5, CAS= 15905-32-5 and for sodium salt of Erythrosine B: formula=C20H6I4Na2O5, CAS= 568-63-8