Difference between revisions of "Copper acetoarsenite"
| (One intermediate revision by one other user not shown) | |||
| Line 1: | Line 1: | ||
| − | [[File:emeraldgr C100x.jpg|thumb|Emerald green]] | + | [[File:emeraldgr C100x.jpg|thumb|Emerald green at 100x (visible light left; UV light right)]] |
== Description == | == Description == | ||
A bright green powder known as the pigment [[emerald green]]. Copper acetoarsenite is prepared by reacting [[sodium arsenite]] with [[copper sulfate]] and [[acetic acid]]. First manufacturered in 1814, it has a brilliant green color that was used in oil and watercolor paints in the 19th century. It is no longer used in artist paints because of its toxicity and poor color permanency. Copper acetoarsenite is currently used as a wood preservative, larvicide and antifouling agent in marine paints. | A bright green powder known as the pigment [[emerald green]]. Copper acetoarsenite is prepared by reacting [[sodium arsenite]] with [[copper sulfate]] and [[acetic acid]]. First manufacturered in 1814, it has a brilliant green color that was used in oil and watercolor paints in the 19th century. It is no longer used in artist paints because of its toxicity and poor color permanency. Copper acetoarsenite is currently used as a wood preservative, larvicide and antifouling agent in marine paints. | ||
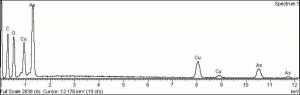
| − | + | [[[SliderGallery rightalign|f394sem.jpg~SEM|f394edsbw.jpg~EDS]]] | |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
emerald green; Pigment Green 21; CI 77410; verde esmeralda (Esp.); vert cendre (Fr.); acetoarsenito di rame (It.); cupric acetoarsenite; copper acetate arsenite; king's green; Paris green; Schweinfurt green; mineral green; imperial green; Mitis green; parrot green; Vienna green; new green; patent green; Emperor green; Kaiser green; meadow green; English green | emerald green; Pigment Green 21; CI 77410; verde esmeralda (Esp.); vert cendre (Fr.); acetoarsenito di rame (It.); cupric acetoarsenite; copper acetate arsenite; king's green; Paris green; Schweinfurt green; mineral green; imperial green; Mitis green; parrot green; Vienna green; new green; patent green; Emperor green; Kaiser green; meadow green; English green | ||
| − | + | == Risks == | |
| + | |||
| + | * Highly toxic by ingestion, inhalation and skin contact. | ||
| − | == | + | ==Physical and Chemical Properties== |
| − | Soluble in mineral acids, ethanol, potassium cyanide. Insoluble in water. Decomposes in alkalis. Darkens in the presence of sulfur or lead compounds. | + | * Soluble in mineral acids, ethanol, potassium cyanide. Insoluble in water. |
| + | * Decomposes in alkalis. | ||
| + | * Darkens in the presence of sulfur or lead compounds. | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 26: | Line 30: | ||
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
Latest revision as of 13:04, 4 July 2022
Description
A bright green powder known as the pigment Emerald green. Copper acetoarsenite is prepared by reacting Sodium arsenite with Copper sulfate and Acetic acid. First manufacturered in 1814, it has a brilliant green color that was used in oil and watercolor paints in the 19th century. It is no longer used in artist paints because of its toxicity and poor color permanency. Copper acetoarsenite is currently used as a wood preservative, larvicide and antifouling agent in marine paints.
Synonyms and Related Terms
emerald green; Pigment Green 21; CI 77410; verde esmeralda (Esp.); vert cendre (Fr.); acetoarsenito di rame (It.); cupric acetoarsenite; copper acetate arsenite; king's green; Paris green; Schweinfurt green; mineral green; imperial green; Mitis green; parrot green; Vienna green; new green; patent green; Emperor green; Kaiser green; meadow green; English green
Risks
- Highly toxic by ingestion, inhalation and skin contact.
Physical and Chemical Properties
- Soluble in mineral acids, ethanol, potassium cyanide. Insoluble in water.
- Decomposes in alkalis.
- Darkens in the presence of sulfur or lead compounds.
| Composition | Cu(C2H3O2)2.3Cu(AsO2)2 |
|---|---|
| Molecular Weight | mol. wt. = 1013.8 |
| Refractive Index | 1.71 - 1.7 |
Resources and Citations
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 2692
- Thomas B. Brill, Light Its Interaction with Art and Antiquities, Plenum Press, New York City, 1980