Difference between revisions of "Lead sulfate"
| (5 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
[[File:521 lead sulfate.jpg|thumb|Lead sulfate]] | [[File:521 lead sulfate.jpg|thumb|Lead sulfate]] | ||
== Description == | == Description == | ||
| − | + | [[File:Leadsulfate C100x.jpg|thumb|Lead sulfate at 100x (visible light left; UV light right)]] | |
| − | A white, heavy powder that is used as a [ | + | A white, heavy powder that is used as a [[pigment]]. Lead sulfate occurs naturally in the mineral anglesite. It is synthesized by adding [[sulfuric acid]] to a lead salt solution. Lead sulfate is used in lithography and in weighting fabrics. It is also used as a paint [[drier]]. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 8: | Line 8: | ||
lead (II) sulfate; Pigment White 3; CI 77630; anglesite (mineral); lead sulphate (Br.); sulfato de plomo (Esp.); Metallweiss (Deut.); Milchweiss (Deut.); Bleisulfat (Deut.); Anglesit (Deut.); sulfate de plomb (Fr.); theiikos molybdos (Gr.); solfato di piombo (It.); loodsulfaat (Ned.); sulfato de chumbo (Port.); white lead; Flemish white; Mulhaus white; Mulhouse white; milk white | lead (II) sulfate; Pigment White 3; CI 77630; anglesite (mineral); lead sulphate (Br.); sulfato de plomo (Esp.); Metallweiss (Deut.); Milchweiss (Deut.); Bleisulfat (Deut.); Anglesit (Deut.); sulfate de plomb (Fr.); theiikos molybdos (Gr.); solfato di piombo (It.); loodsulfaat (Ned.); sulfato de chumbo (Port.); white lead; Flemish white; Mulhaus white; Mulhouse white; milk white | ||
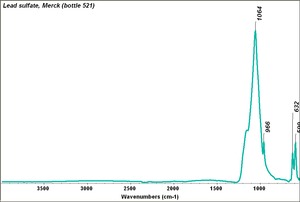
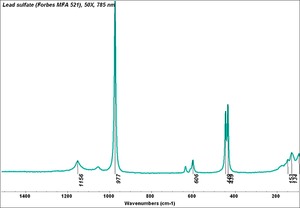
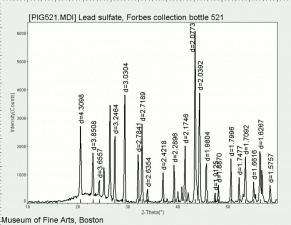
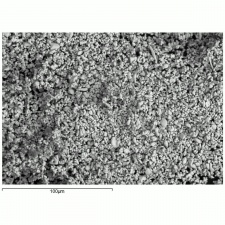
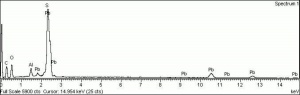
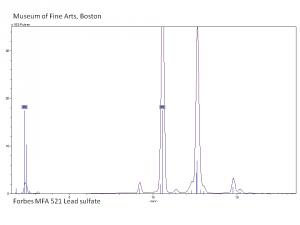
| − | [[[SliderGallery rightalign|MFA | + | [[[SliderGallery rightalign|Lead sulfate 521.TIF~FTIR (MFA)|Lead sulfate (Forbes MFA 521), 50X, 785 nm copy.tif~Raman (MFA)|PIG521.jpg~XRD|f521sem.jpg~SEM|f521edsbw.jpg~EDS|Slide17_F521.PNG~XRF]]] |
| + | == Risks == | ||
| − | == | + | * Toxic by inhalation or ingestion. |
| + | * Noncombustible. | ||
| + | * Skin contact may cause irritation or ulcers. | ||
| + | * Carcinogen, teratogen, suspected mutagen. | ||
| + | * ThermoFisher: [https://www.fishersci.com/store/msds?partNumber=AA1073009&productDescription=LEAD%28II%29+SULFATE+99.999%25+10G&vendorId=VN00024248&countryCode=US&language=en SDS] | ||
| + | == Physical and Chemical Properties == | ||
Soluble in sodium hydroxide solution, concentrated hydriodic acid. Slightly solution in hot water. Insoluble in ethanol. | Soluble in sodium hydroxide solution, concentrated hydriodic acid. Slightly solution in hot water. Insoluble in ethanol. | ||
| Line 28: | Line 34: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 1170 | + | | 1170 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 6.12-6.39 | + | | 6.12-6.39 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 39: | Line 45: | ||
| 1.878; 1.883; 1.895 | | 1.878; 1.883; 1.895 | ||
|} | |} | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
== Comparisons == | == Comparisons == | ||
| Line 52: | Line 50: | ||
[[media:download_file_514.pdf|Characteristics of Common White Pigments]] | [[media:download_file_514.pdf|Characteristics of Common White Pigments]] | ||
| − | + | == Resources and Citations == | |
| − | + | * M-C. Corbeil, P.J. Sirois, E.A. Moffatt, "The use of a white pigment patented by Freeman by Tom Thomson and the Group of Seven" preprints ICOM, Lyons, 1999. p.369. | |
| − | == | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 444 | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 444 | ||
| Line 69: | Line 59: | ||
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 5444 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 5444 | ||
| − | * Wikipedia | + | * Wikipedia: http://en.wikipedia.org/wiki/Lead_sulfate (Accessed Sept. 7, 2005) |
* Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, ''Technology and Conservation'', Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985 | * Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, ''Technology and Conservation'', Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985 | ||
Latest revision as of 08:49, 7 October 2022
Description
A white, heavy powder that is used as a Pigment. Lead sulfate occurs naturally in the mineral anglesite. It is synthesized by adding Sulfuric acid to a lead salt solution. Lead sulfate is used in lithography and in weighting fabrics. It is also used as a paint Drier.
Synonyms and Related Terms
lead (II) sulfate; Pigment White 3; CI 77630; anglesite (mineral); lead sulphate (Br.); sulfato de plomo (Esp.); Metallweiss (Deut.); Milchweiss (Deut.); Bleisulfat (Deut.); Anglesit (Deut.); sulfate de plomb (Fr.); theiikos molybdos (Gr.); solfato di piombo (It.); loodsulfaat (Ned.); sulfato de chumbo (Port.); white lead; Flemish white; Mulhaus white; Mulhouse white; milk white
Risks
- Toxic by inhalation or ingestion.
- Noncombustible.
- Skin contact may cause irritation or ulcers.
- Carcinogen, teratogen, suspected mutagen.
- ThermoFisher: SDS
Physical and Chemical Properties
Soluble in sodium hydroxide solution, concentrated hydriodic acid. Slightly solution in hot water. Insoluble in ethanol.
Transparent colorless particles showing high relief and moderate birefringence under crossed polars
| Composition | PbSO4 |
|---|---|
| CAS | 7446-14-2 |
| Mohs Hardness | 2.75 |
| Melting Point | 1170 C |
| Density | 6.12-6.39 g/ml |
| Molecular Weight | mol. wt. = 303.28 |
| Refractive Index | 1.878; 1.883; 1.895 |
Comparisons
Characteristics of Common White Pigments
Resources and Citations
- M-C. Corbeil, P.J. Sirois, E.A. Moffatt, "The use of a white pigment patented by Freeman by Tom Thomson and the Group of Seven" preprints ICOM, Lyons, 1999. p.369.
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 444
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 5444
- Wikipedia: http://en.wikipedia.org/wiki/Lead_sulfate (Accessed Sept. 7, 2005)
- Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985