Difference between revisions of "Titanium dioxide"
| (23 intermediate revisions by 6 users not shown) | |||
| Line 1: | Line 1: | ||
| + | [[File:Rutilated quartz-SC268387 (1).jpg|thumb|Rutilated quartz<br>MFA# 01.7556]] | ||
[[File:pr21272rutile.jpg|thumb|Rutile]] | [[File:pr21272rutile.jpg|thumb|Rutile]] | ||
| + | [[File:536 titanium oxide.jpg|thumb|Titanium dioxide pigment MFA bottle 536]] | ||
== Description == | == Description == | ||
| − | + | Titanium dioxide occurs naturally in three crystalline forms: [[anatase]], [[rutile]] and [[brookite]], with rutile being the most abundant. In mineral form, titanium dioxide is often deeply coloured due to elemental impurities. Ground mineral rutile found some use as a colored pigment, but difficulties in grinding result in coarse particles with a different morphology than that of synthetic material. Naturally occurring anatase and brookite are not used for pigment manufacture. Both anatase and rutile have been synthesized since the early 20th century for use as white pigments, generally using processes based on either sulfuric acid or chlorine. The pigments are used in applications including: colorants and opacifiers in paints, pastels, inks, enamels, ceramics, glass, rubber and plastics; fillers and coatings in book, fine and photographic paper; and as coatings, delustrants and surface treatments in the textile and leather industries. They are nontoxic and can be used in foods and pharmaceuticals; their ultraviolet absorption properties have led to applications in sunscreen and lotions. Titanium dioxide is also used as a white reference material in many instruments for optical measurements or color spectrometry. Rutile can be made into synthetic gemstones of varying color and has use as a coating in the manufacture of welding rods. | |
| − | [[ | + | The earliest uses of a synthetic titanium oxide, not of pigmentary quality, were during the late 1800s as opacifiers and additives to increase acid resistance in glazes and vitreous enamels. A blue porcelain glaze using hydrous titanic oxide was described in 1841. Titanium compounds of all kinds were investigated for use in the late 1800s in the textile industry, including titanium oxide as a mordant for wool, also not the calcined white pigment. From about 1908 onward, researchers in Norway and in the United States who were seeking to find a use for titanium-rich ore such as [[ilmenite]] (iron titanium oxide) noted that titanium dioxide showed promise as a pigment due to its high hiding power. During development of a viable manufacturing method, small experimental batches of pigment were produced of various compositions and ranging in color from reddish yellow to off-white. Commercial manufacturing of anatase-based pigments began in Norway in 1918 (Titan Company A/S), and in the United States (Titanium Pigment Company) ramping up from 1916 to full production in 1919. Anatase pigments were developed first, and most of these early products were composites with barium sulfate, some (in Norway) also with calcium phosphate; pure pigments with 98% titanium dioxide were only introduced in 1923 in France and 1926 in the United States (an 83% pure product in Norway was replaced by a composite in the first year of production: for more detail on history see [[anatase]]). Because anatase converts to rutile at high temperatures, calcination of the early pigments could result in up to 20% of coarse, gritty rutile. Rutile pigments, which have better hiding power and weathering properties than antase products, were produced experimentally on a laboratory scale in 1931 in Czechoslovakia, Germany and the United States, but the two methods which resulted in industrial scale production were not developed until 1937; commercial introduction of these pigments occurred from 1938 onward. An alternate process for the production of rutile pigment using chlorine was introduced on a pilot plant scale in the United States in 1948 (E.I. du Pont de Nemours and Co.). The chloride process was briefly used to produce anatase pigment in the United States (1975 to 1985, du Pont), which was mainly used in the paper industry; chloride process anatase contained a significant amount of rutile. Due to differences in starting material and method, chloride process pigment may be able to be distinguished from that made by the sulfate process on the basis of trace elements (see [[rutile]]). |
| + | |||
| + | From its earliest manufacture, many compounds were coprecipitated with, deposited on, or admixed with titanium dioxide to improve properties or reduce cost, including compounds of barium, calcium, aluminum, silicon, magnesium, antimony, zinc, lead and others. Colored titanium dioxide pigments can be made by absorbing an organic colorant onto titanium dioxide. | ||
| + | |||
| + | Commercial paint manufacturers did not immediately convert from traditional white pigments to titanium dioxide (due to higher cost, somewhat unreliable color, limited supplies, and general inertia); however, during the 1920s many countries introduced laws to restrict the use of toxic lead white, which gave impetus to the change. Artists' color makers were also slow to introduce titanium dioxide in their palettes: Societe Bourgeois in 1925 (pure anatase); Societe Lefranc in 1927 (pure anatase); Winsor & Newton in 1928; H. Schminke and Company in the early 1930s; Koninklijke Talens BV in 1937; Sadolin and Holmblad A/S circa 1938; George Rowney and Co. and Danacolors, Inc. after 1938. Artists' manuals reflect the gradual acceptance of the new products through the 1930s. The "titanium white" products sold to artists varied considerably in composition and were often admixed with other white pigments and fillers. | ||
| + | |||
| + | Experiments into printing on damask and cotton using anatase pigment in albumin, viscose and acetyl cellulose media were being undertaken by 1923 and it was used in other applications such as delustering synthetic textiles such as cellulose acetate and rayon. The white anatase pigments were used in inks as colorant and opacifier in the 1920s. The pigment was mentioned in reference to wallpaper in 1927, and from 1931 to 1936, the composite and pure anatase pigments were tried in most types of paper, including photographic paper (to replace barium sulfate) in 1934. Use as a colorant in linoleum was patented in 1917, and by 1921 it proved to be a beneficial additive to rubber. | ||
| + | |||
| + | Although both anatase and rutile titanium dioxide pigments are lightfast, exposure to light can cause a phototropic reversible blue-gray discoloration on the surface in some circumstances. The tendency of the early pigments to blush or redden on exposure to bright light was attributable to impurities and was eliminated by improved processing. Both anatase and rutile are strong ultraviolet absorbers, and they are photochemically active. The tendency to chalk, a loss of gloss and mass in the paint medium which leaves loose pigment particles on the surface, was a disadvantage of the early titanium dioxide pigments; rutile is less prone to photoreactivity than anatase. Coatings and stabilizing elements added to the titanium dioxide lattice in various grades of pigment have been used to reduce the photoactivity. The pigments can cause chemical reactions in associated media or colorants; adding zinc oxide to artists' paint mixtures containing sensitive materials such as azo dyes or basic dyestuffs such as madder lake can reduce fading. Cellulosic textile fibers delustered with titanium dioxide, including rayon, nylon and cotton, can undergo accelerated deterioration due to the presence of titanium dioxide pigment. It should be noted that, depending on the application, titanium dioxide pigment can reduce the damaging effects of light in a medium which would photodegrade readily in the absence of pigment. In cases where the pigment used in a coating, the effect can be protective for material below. | ||
| + | |||
| + | Titanium dioxide pigments are noted for their extremely small particle size, typically less than 0.4 micrometers. Composite pigments generally have a larger average particle size. In the sulfate process, the size of the titanium dioxide particles is not controlled by grinding, but is determined during the hydrolysis/precipitation and the calcination steps. Formation of a useful pigment requires calcination, usually in a rotary oven at around 900C over a period of hours. Grinding serves to reduce the number of crystallites in the sintered particle aggregates. Crystal structure (anatase or rutile) is adjustable during the hydrolysis/precipitation step (using various additives) and during calcination. In the chloride process, based on gaseous reactions, crystal structure and particle size are determined during the final brief step of vapor phase oxidation of titanium tetrachloride, in milliseconds at around 1200C, generally in the presence of rutilization catalysts (e.g., aluminum chloride) or other additives. | ||
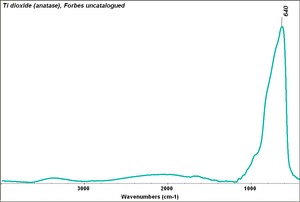
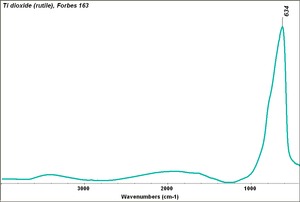
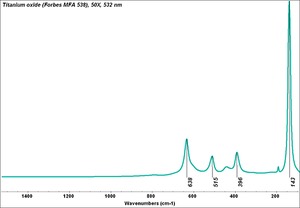
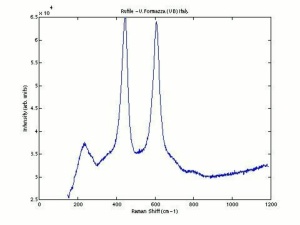
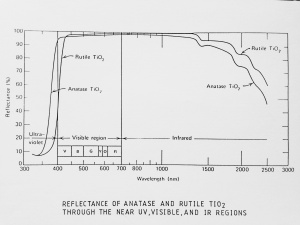
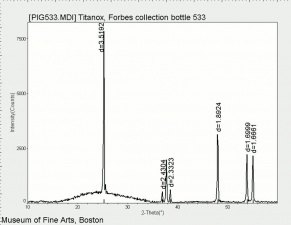
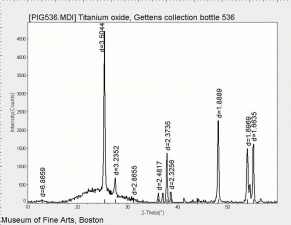
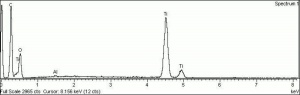
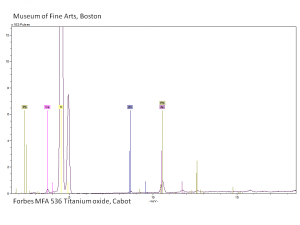
| + | [[[SliderGallery rightalign|Ti dioxide (anatase), Forbes.TIF~FTIR (MFA)|Ti dioxide (rutile), Forbes 163.TIF~FTIR (MFA)|Titanium oxide (Forbes MFA 538), 50X, 532 nm copy.tif~Raman Anatase (MFA)|Rutileitaly1.jpg~Raman Rutile (UCL)|Reflectance.jpg~Reflectance|PIG533.jpg~XRD|PIG536.jpg~XRD|f533sem.jpg~SEM|f536sem.jpg~SEM|f533edsbw.jpg~EDS|Slide23_F536.PNG~XRF]]] | ||
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| − | titanium white; rutile; anatase; brookite; Pigment White 6; CI 77891; dioxyde de titane (Fr.); Titandioxid (Deut., Sven.); bianco di titanio (It.); dióxido de titanio (Esp.); titandioksid (Nor.); dióxido de titânio (Port.); titania; titanic anhydride; titanic acid anhydride; titanic oxide; Unitane; Titanox | + | titanium white; rutile; anatase; brookite; Pigment White 6; CI 77891; dioxyde de titane (Fr.); Titandioxid (Deut., Sven.); bianco di titanio (It.); dióxido de titanio (Esp.); titandioksid (Nor.); dióxido de titânio (Port.); titania; titanic anhydride; titanic acid anhydride; titanic oxide; Unitane; Titanox; Kronos |
| − | + | == Risks == | |
| − | + | Nontoxic. Noncombustible. | |
| + | |||
| + | No significant hazards. | ||
| − | + | Fisher Scientific: [https://beta-static.fishersci.com/content/dam/fishersci/en_US/documents/programs/education/regulatory-documents/sds/chemicals/chemicals-t/S25818.pdf SDS] | |
| + | == Physical and Chemical Properties == | ||
| − | + | Particle size (pigment, including composites) = 0.14-0.8 micrometers. | |
| − | + | Chemically inert, insoluble in water, organic solvents, aqueous alkalis; can be dissolved in sulfuric or hydrofluoric acid; slow to dry in oil without additives or surface treatment. | |
{| class="wikitable" | {| class="wikitable" | ||
| Line 24: | Line 40: | ||
| TiO2 | | TiO2 | ||
|- | |- | ||
| − | ! scope="row"| | + | ! scope="row"| Chemical Abstracts Service |
| 13463-67-7 | | 13463-67-7 | ||
| + | |- | ||
| + | ! scope="row"| Molecular Weight | ||
| + | | 79.9 | ||
|- | |- | ||
! scope="row"| Mohs Hardness | ! scope="row"| Mohs Hardness | ||
| − | | 6 | + | | anatase 5.5-6 |
| + | | rutile 6-6.5 | ||
| + | | brookite 5.5-6 | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | | + | | anatase n/a (significant conversion to rutile above about 700C; anatase pigments range from 400-1200C) |
| + | | rutile 1825C | ||
| + | | brookite n/a (converts to rutile) | ||
|- | |- | ||
| − | ! scope="row"| Density | + | ! scope="row"| Density (Calc.) |
| − | | | + | | anatase 3.89 |
| − | | | + | | rutile 4.25 |
| − | + | | brookite 4.13 | |
| − | |||
|- | |- | ||
! scope="row"| Refractive Index | ! scope="row"| Refractive Index | ||
| − | | 2. | + | | anatase: mineral 2.54-2.56; pure pigment (e.g., Titanox A) 2.3-2.65; composite (e.g., Titanox B/C) 1.8-2.3 |
| + | | rutile: mineral 2.61-2.9; pure pigment ~2.7; composite ~1.98 | ||
| + | | brookite: 2.58-2.74 | ||
|} | |} | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
== Comparisons == | == Comparisons == | ||
| Line 63: | Line 75: | ||
[[media:download_file_517.pdf|Characteristics of Common White Pigments]] | [[media:download_file_517.pdf|Characteristics of Common White Pigments]] | ||
| + | ==Resources and Citations== | ||
| + | * M.Laver, "Titanium Dioxide Whites", ''Artists Pigments'', Volume 3, E. West FitzHugh (ed.), Oxford University Press: Oxford, 1997.-In Norway, production of TiO2 patented in 1913, and pigment production in November 1918 (Titan Co. In U.S., patent applications filed in 1912 and production of a composite pigment began in 1916. | ||
| − | + | * Walter C. McCrone, "Polarized Light Microscopy in Conservation: A Personal Perspective" ''JAIC'' 33(2):101-14, 1994. (contains a table of dates on the history of titanium white as a pigment) | |
| − | |||
* ''The Dictionary of Art'', Grove's Dictionaries Inc., New York, 1996 Comment: "Pigments" | * ''The Dictionary of Art'', Grove's Dictionaries Inc., New York, 1996 Comment: "Pigments" | ||
| − | + | ||
| − | |||
| − | |||
* R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 | * R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 | ||
| Line 93: | Line 104: | ||
* M. de Keijzer, 'A survey of red and yellow modern synthetic organic artists pigments discovered in the 20th century and used in oil colors', ''ICOM Preprints'' Lyons, France, Getty Conservation Institute, Los Angeles, p. 369, 1999 | * M. de Keijzer, 'A survey of red and yellow modern synthetic organic artists pigments discovered in the 20th century and used in oil colors', ''ICOM Preprints'' Lyons, France, Getty Conservation Institute, Los Angeles, p. 369, 1999 | ||
| − | * Wikipedia | + | * Wikipedia: http://en.wikipedia.org/wiki/Titanium_dioxide (Accessed Sept. 17, 2005) |
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
| Line 101: | Line 112: | ||
* Book and Paper Group, ''Paper Conservation Catalog'', AIC, 1984, 1989 | * Book and Paper Group, ''Paper Conservation Catalog'', AIC, 1984, 1989 | ||
| − | * | + | * Submission by N. Marco 8/25/05. |
| − | * | + | * Pigments Through the Ages -http://webexhibits.org/pigments/indiv/overview/tiwhite.html |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 09:21, 29 August 2020
Description
Titanium dioxide occurs naturally in three crystalline forms: Anatase, Rutile and Brookite, with rutile being the most abundant. In mineral form, titanium dioxide is often deeply coloured due to elemental impurities. Ground mineral rutile found some use as a colored pigment, but difficulties in grinding result in coarse particles with a different morphology than that of synthetic material. Naturally occurring anatase and brookite are not used for pigment manufacture. Both anatase and rutile have been synthesized since the early 20th century for use as white pigments, generally using processes based on either sulfuric acid or chlorine. The pigments are used in applications including: colorants and opacifiers in paints, pastels, inks, enamels, ceramics, glass, rubber and plastics; fillers and coatings in book, fine and photographic paper; and as coatings, delustrants and surface treatments in the textile and leather industries. They are nontoxic and can be used in foods and pharmaceuticals; their ultraviolet absorption properties have led to applications in sunscreen and lotions. Titanium dioxide is also used as a white reference material in many instruments for optical measurements or color spectrometry. Rutile can be made into synthetic gemstones of varying color and has use as a coating in the manufacture of welding rods.
The earliest uses of a synthetic titanium oxide, not of pigmentary quality, were during the late 1800s as opacifiers and additives to increase acid resistance in glazes and vitreous enamels. A blue porcelain glaze using hydrous titanic oxide was described in 1841. Titanium compounds of all kinds were investigated for use in the late 1800s in the textile industry, including titanium oxide as a mordant for wool, also not the calcined white pigment. From about 1908 onward, researchers in Norway and in the United States who were seeking to find a use for titanium-rich ore such as Ilmenite (iron titanium oxide) noted that titanium dioxide showed promise as a pigment due to its high hiding power. During development of a viable manufacturing method, small experimental batches of pigment were produced of various compositions and ranging in color from reddish yellow to off-white. Commercial manufacturing of anatase-based pigments began in Norway in 1918 (Titan Company A/S), and in the United States (Titanium Pigment Company) ramping up from 1916 to full production in 1919. Anatase pigments were developed first, and most of these early products were composites with barium sulfate, some (in Norway) also with calcium phosphate; pure pigments with 98% titanium dioxide were only introduced in 1923 in France and 1926 in the United States (an 83% pure product in Norway was replaced by a composite in the first year of production: for more detail on history see Anatase). Because anatase converts to rutile at high temperatures, calcination of the early pigments could result in up to 20% of coarse, gritty rutile. Rutile pigments, which have better hiding power and weathering properties than antase products, were produced experimentally on a laboratory scale in 1931 in Czechoslovakia, Germany and the United States, but the two methods which resulted in industrial scale production were not developed until 1937; commercial introduction of these pigments occurred from 1938 onward. An alternate process for the production of rutile pigment using chlorine was introduced on a pilot plant scale in the United States in 1948 (E.I. du Pont de Nemours and Co.). The chloride process was briefly used to produce anatase pigment in the United States (1975 to 1985, du Pont), which was mainly used in the paper industry; chloride process anatase contained a significant amount of rutile. Due to differences in starting material and method, chloride process pigment may be able to be distinguished from that made by the sulfate process on the basis of trace elements (see Rutile).
From its earliest manufacture, many compounds were coprecipitated with, deposited on, or admixed with titanium dioxide to improve properties or reduce cost, including compounds of barium, calcium, aluminum, silicon, magnesium, antimony, zinc, lead and others. Colored titanium dioxide pigments can be made by absorbing an organic colorant onto titanium dioxide.
Commercial paint manufacturers did not immediately convert from traditional white pigments to titanium dioxide (due to higher cost, somewhat unreliable color, limited supplies, and general inertia); however, during the 1920s many countries introduced laws to restrict the use of toxic lead white, which gave impetus to the change. Artists' color makers were also slow to introduce titanium dioxide in their palettes: Societe Bourgeois in 1925 (pure anatase); Societe Lefranc in 1927 (pure anatase); Winsor & Newton in 1928; H. Schminke and Company in the early 1930s; Koninklijke Talens BV in 1937; Sadolin and Holmblad A/S circa 1938; George Rowney and Co. and Danacolors, Inc. after 1938. Artists' manuals reflect the gradual acceptance of the new products through the 1930s. The "titanium white" products sold to artists varied considerably in composition and were often admixed with other white pigments and fillers.
Experiments into printing on damask and cotton using anatase pigment in albumin, viscose and acetyl cellulose media were being undertaken by 1923 and it was used in other applications such as delustering synthetic textiles such as cellulose acetate and rayon. The white anatase pigments were used in inks as colorant and opacifier in the 1920s. The pigment was mentioned in reference to wallpaper in 1927, and from 1931 to 1936, the composite and pure anatase pigments were tried in most types of paper, including photographic paper (to replace barium sulfate) in 1934. Use as a colorant in linoleum was patented in 1917, and by 1921 it proved to be a beneficial additive to rubber.
Although both anatase and rutile titanium dioxide pigments are lightfast, exposure to light can cause a phototropic reversible blue-gray discoloration on the surface in some circumstances. The tendency of the early pigments to blush or redden on exposure to bright light was attributable to impurities and was eliminated by improved processing. Both anatase and rutile are strong ultraviolet absorbers, and they are photochemically active. The tendency to chalk, a loss of gloss and mass in the paint medium which leaves loose pigment particles on the surface, was a disadvantage of the early titanium dioxide pigments; rutile is less prone to photoreactivity than anatase. Coatings and stabilizing elements added to the titanium dioxide lattice in various grades of pigment have been used to reduce the photoactivity. The pigments can cause chemical reactions in associated media or colorants; adding zinc oxide to artists' paint mixtures containing sensitive materials such as azo dyes or basic dyestuffs such as madder lake can reduce fading. Cellulosic textile fibers delustered with titanium dioxide, including rayon, nylon and cotton, can undergo accelerated deterioration due to the presence of titanium dioxide pigment. It should be noted that, depending on the application, titanium dioxide pigment can reduce the damaging effects of light in a medium which would photodegrade readily in the absence of pigment. In cases where the pigment used in a coating, the effect can be protective for material below.
Titanium dioxide pigments are noted for their extremely small particle size, typically less than 0.4 micrometers. Composite pigments generally have a larger average particle size. In the sulfate process, the size of the titanium dioxide particles is not controlled by grinding, but is determined during the hydrolysis/precipitation and the calcination steps. Formation of a useful pigment requires calcination, usually in a rotary oven at around 900C over a period of hours. Grinding serves to reduce the number of crystallites in the sintered particle aggregates. Crystal structure (anatase or rutile) is adjustable during the hydrolysis/precipitation step (using various additives) and during calcination. In the chloride process, based on gaseous reactions, crystal structure and particle size are determined during the final brief step of vapor phase oxidation of titanium tetrachloride, in milliseconds at around 1200C, generally in the presence of rutilization catalysts (e.g., aluminum chloride) or other additives.
Synonyms and Related Terms
titanium white; rutile; anatase; brookite; Pigment White 6; CI 77891; dioxyde de titane (Fr.); Titandioxid (Deut., Sven.); bianco di titanio (It.); dióxido de titanio (Esp.); titandioksid (Nor.); dióxido de titânio (Port.); titania; titanic anhydride; titanic acid anhydride; titanic oxide; Unitane; Titanox; Kronos
Risks
Nontoxic. Noncombustible.
No significant hazards.
Fisher Scientific: SDS
Physical and Chemical Properties
Particle size (pigment, including composites) = 0.14-0.8 micrometers.
Chemically inert, insoluble in water, organic solvents, aqueous alkalis; can be dissolved in sulfuric or hydrofluoric acid; slow to dry in oil without additives or surface treatment.
| Composition | TiO2 | ||
|---|---|---|---|
| Chemical Abstracts Service | 13463-67-7 | ||
| Molecular Weight | 79.9 | ||
| Mohs Hardness | anatase 5.5-6 | rutile 6-6.5 | brookite 5.5-6 |
| Melting Point | anatase n/a (significant conversion to rutile above about 700C; anatase pigments range from 400-1200C) | rutile 1825C | brookite n/a (converts to rutile) |
| Density (Calc.) | anatase 3.89 | rutile 4.25 | brookite 4.13 |
| Refractive Index | anatase: mineral 2.54-2.56; pure pigment (e.g., Titanox A) 2.3-2.65; composite (e.g., Titanox B/C) 1.8-2.3 | rutile: mineral 2.61-2.9; pure pigment ~2.7; composite ~1.98 | brookite: 2.58-2.74 |
Comparisons
Properties of Common Abrasives
Natural and Simulated Diamonds
Characteristics of Common White Pigments
Resources and Citations
- M.Laver, "Titanium Dioxide Whites", Artists Pigments, Volume 3, E. West FitzHugh (ed.), Oxford University Press: Oxford, 1997.-In Norway, production of TiO2 patented in 1913, and pigment production in November 1918 (Titan Co. In U.S., patent applications filed in 1912 and production of a composite pigment began in 1916.
- Walter C. McCrone, "Polarized Light Microscopy in Conservation: A Personal Perspective" JAIC 33(2):101-14, 1994. (contains a table of dates on the history of titanium white as a pigment)
- The Dictionary of Art, Grove's Dictionaries Inc., New York, 1996 Comment: "Pigments"
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982
- Dictionary of Building Preservation, Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996 Comment: Pigment available in 1918
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 9612
- Reed Kay, The Painter's Guide To Studio Methods and Materials, Prentice-Hall, Inc., Englewood Cliffs, NJ, 1983
- Hoechst Celanese Corporation, Dictionary of Fiber & Textile Technology (older version called Man-made Fiber and Textile Dictionary, 1965), Hoechst Celanese Corporation, Charlotte NC, 1990
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- C.W.Chesterman, K.E.Lowe, Audubon Society Field Guide to North American Rocks and Minerals, Alfred A. Knopf, New York, 1979 Comment: density=4.2-4.3, hardness=6.0-6.5
- Thomas B. Brill, Light Its Interaction with Art and Antiquities, Plenum Press, New York City, 1980
- M. de Keijzer, 'A survey of red and yellow modern synthetic organic artists pigments discovered in the 20th century and used in oil colors', ICOM Preprints Lyons, France, Getty Conservation Institute, Los Angeles, p. 369, 1999
- Wikipedia: http://en.wikipedia.org/wiki/Titanium_dioxide (Accessed Sept. 17, 2005)
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985 Comment: Artists pigments sold in mid 1920s
- Book and Paper Group, Paper Conservation Catalog, AIC, 1984, 1989
- Submission by N. Marco 8/25/05.
- Pigments Through the Ages -http://webexhibits.org/pigments/indiv/overview/tiwhite.html