Difference between revisions of "Chrome yellow"
| (7 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
[[File:306 chrome yellow.jpg|thumb|Chrome yellow]] | [[File:306 chrome yellow.jpg|thumb|Chrome yellow]] | ||
== Description == | == Description == | ||
| − | + | [[File:Cryellow C100x.jpg|thumb|Chromium yellow at 100 xx (visible light on left / UV light on right)]] | |
| + | [[File:12_Chrome_yellow_200X.jpg|thumb|Chrome yellow at 200x]] | ||
A toxic yellow artist's pigment containing [[lead chromate]] sometimes mixed with [[lead sulfate]]. Lead chromate can range in shade from lemon yellow to orange depending on its particle size, hydration state, and percent lead chromate. Chrome yellow, which came on the market in early 1800s, is permanent to visible light, but can darken with exposure to [[ultraviolet radiation|UV radiation]] or [[hydrogen sulfide]]. Chrome yellow is used in industrial paints, some artist's paints and ceramic glazes. | A toxic yellow artist's pigment containing [[lead chromate]] sometimes mixed with [[lead sulfate]]. Lead chromate can range in shade from lemon yellow to orange depending on its particle size, hydration state, and percent lead chromate. Chrome yellow, which came on the market in early 1800s, is permanent to visible light, but can darken with exposure to [[ultraviolet radiation|UV radiation]] or [[hydrogen sulfide]]. Chrome yellow is used in industrial paints, some artist's paints and ceramic glazes. | ||
| − | Other yellow chromate pigments are sometimes also called chrome yellow. [[Strontium chromate]], [[zinc chromate]], and [[barium chromate]] are pale yellow pigments that are often mixed and called lemon yellow. Strontium chromate has more hiding power than the barium chromate. [[Zinc yellow]] is synthetically prepared zinc chromate. The pure material is stable and is used in oil and watercolor paints. | + | Other yellow chromate pigments are sometimes also called chrome yellow. [[Strontium chromate]], [[zinc yellow|zinc chromate]], and [[barium chromate]] are pale yellow pigments that are often mixed and called lemon yellow. Strontium chromate has more hiding power than the barium chromate. [[Zinc yellow]] is synthetically prepared zinc chromate. The pure material is stable and is used in oil and watercolor paints. |
| − | |||
| − | |||
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 12: | Line 11: | ||
Pigment Yellow 34; CI 77600; Chromgelb (Deut.); jaune de chrôme (Fr.); giallo cromo (It.); amarillo de cromo (Esp.); amarelo de crómio (Port.); Paris yellow; king's yellow; Vienna yellow; lemon yellow; jonquil chrome yellow; Cologne yellow; Leipzig yellow | Pigment Yellow 34; CI 77600; Chromgelb (Deut.); jaune de chrôme (Fr.); giallo cromo (It.); amarillo de cromo (Esp.); amarelo de crómio (Port.); Paris yellow; king's yellow; Vienna yellow; lemon yellow; jonquil chrome yellow; Cologne yellow; Leipzig yellow | ||
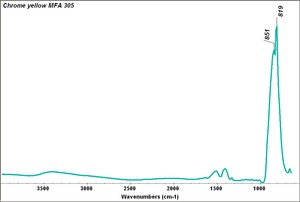
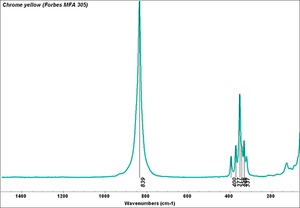
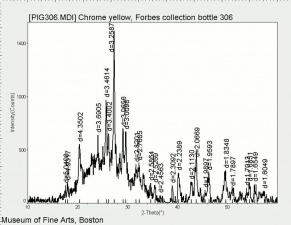
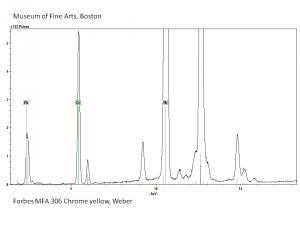
| − | [[[SliderGallery rightalign| | + | [[[SliderGallery rightalign|Chrome yellow 305.TIF~FTIR (MFA)|Chrome yellow (Forbes MFA 305) resize.tif~Raman (MFA)|PIG306.jpg~XRD|f306sem.jpg~SEM|f306edsbw.jpg~EDS|Slide16 FC306.PNG~XRF]]] |
| + | |||
| + | == Risks == | ||
| − | + | * Toxic by inhalation or ingestion. | |
| + | * Skin contact may cause irritation or ulcers. Carcinogen, teratogen, suspected mutagen. | ||
| + | * Discolored by heat, UV light and sulfur fumes. | ||
| + | * SigmaAldrich: [https://www.sigmaaldrich.com/US/en/product/aldrich/s475017 SDS] | ||
| − | + | == Physical and Chemical Properties == | |
| − | High birefringence. Monoclinic prism crystals. | + | * Soluble in strong acids and alkalis. Insoluble in water. |
| + | * High birefringence. | ||
| + | * Monoclinic prism crystals. | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 29: | Line 35: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 844 | + | | 844 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 5.96 - 6.3 | + | | 5.96 - 6.3 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 41: | Line 47: | ||
|} | |} | ||
| − | == | + | == Resources and Citations == |
| + | * H. Kuhn, M.Curran, "Chrome Yellow and Other Chromate Pigments", ''Artists Pigments'', Volume 1, R. Feller (ed.), Cambridge University Press: Cambridge, 1986. | ||
| − | + | * Pigments Through the Ages: [http://webexhibits.org/pigments/indiv/overview/cryellow.html Chrome yellow] | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 Comment: density = 5.96 and ref. index = 2.31; 2.49 | * R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 Comment: density = 5.96 and ref. index = 2.31; 2.49 | ||
| Line 81: | Line 69: | ||
* ''Dictionary of Building Preservation'', Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996 | * ''Dictionary of Building Preservation'', Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996 | ||
| − | |||
| − | |||
* Thomas B. Brill, ''Light Its Interaction with Art and Antiquities'', Plenum Press, New York City, 1980 | * Thomas B. Brill, ''Light Its Interaction with Art and Antiquities'', Plenum Press, New York City, 1980 | ||
Latest revision as of 13:30, 29 May 2022
Description
A toxic yellow artist's pigment containing Lead chromate sometimes mixed with Lead sulfate. Lead chromate can range in shade from lemon yellow to orange depending on its particle size, hydration state, and percent lead chromate. Chrome yellow, which came on the market in early 1800s, is permanent to visible light, but can darken with exposure to UV radiation or Hydrogen sulfide. Chrome yellow is used in industrial paints, some artist's paints and ceramic glazes.
Other yellow chromate pigments are sometimes also called chrome yellow. Strontium chromate, zinc chromate, and Barium chromate are pale yellow pigments that are often mixed and called lemon yellow. Strontium chromate has more hiding power than the barium chromate. Zinc yellow is synthetically prepared zinc chromate. The pure material is stable and is used in oil and watercolor paints.
Synonyms and Related Terms
Pigment Yellow 34; CI 77600; Chromgelb (Deut.); jaune de chrôme (Fr.); giallo cromo (It.); amarillo de cromo (Esp.); amarelo de crómio (Port.); Paris yellow; king's yellow; Vienna yellow; lemon yellow; jonquil chrome yellow; Cologne yellow; Leipzig yellow
Risks
- Toxic by inhalation or ingestion.
- Skin contact may cause irritation or ulcers. Carcinogen, teratogen, suspected mutagen.
- Discolored by heat, UV light and sulfur fumes.
- SigmaAldrich: SDS
Physical and Chemical Properties
- Soluble in strong acids and alkalis. Insoluble in water.
- High birefringence.
- Monoclinic prism crystals.
| Composition | PbCrO4 |
|---|---|
| CAS | 7758-97-6 |
| Melting Point | 844 C |
| Density | 5.96 - 6.3 g/ml |
| Molecular Weight | mol. wt. = 323.2 |
| Refractive Index | 2.31; 2.49 |
Resources and Citations
- H. Kuhn, M.Curran, "Chrome Yellow and Other Chromate Pigments", Artists Pigments, Volume 1, R. Feller (ed.), Cambridge University Press: Cambridge, 1986.
- Pigments Through the Ages: Chrome yellow
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966 Comment: density = 5.96 and ref. index = 2.31; 2.49
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 445
- Reed Kay, The Painter's Guide To Studio Methods and Materials, Prentice-Hall, Inc., Englewood Cliffs, NJ, 1983
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- R.D. Harley, Artists' Pigments c. 1600-1835, Butterworth Scientific, London, 1982
- Dictionary of Building Preservation, Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996
- Thomas B. Brill, Light Its Interaction with Art and Antiquities, Plenum Press, New York City, 1980
- The Dictionary of Art, Grove's Dictionaries Inc., New York, 1996 Comment: "Pigments"
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983
- Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000