Difference between revisions of "Chromium trioxide"
| (One intermediate revision by one other user not shown) | |||
| Line 2: | Line 2: | ||
Dark, violet-red deliquescent crystals. Chromium trioxide is normally used as an aqueous solution which is commonly called [[chromic acid]]. Chromic acid is made by mixing [[potassium dichromate]] and an acid such as [[hydrochloric acid]] or [[sulfuric acid]]. It is an extremely strong oxidizing agent that will react violently with any organic compound. For many years, chromic acid was used in chemical laboratories cleaning baths to remove all traces of organic residues from glassware (see [[Beckmann mixture]]). Chromic acid is also used for chrome plating baths, engraving etching, tanning, and as a colorant in glass and ceramics. | Dark, violet-red deliquescent crystals. Chromium trioxide is normally used as an aqueous solution which is commonly called [[chromic acid]]. Chromic acid is made by mixing [[potassium dichromate]] and an acid such as [[hydrochloric acid]] or [[sulfuric acid]]. It is an extremely strong oxidizing agent that will react violently with any organic compound. For many years, chromic acid was used in chemical laboratories cleaning baths to remove all traces of organic residues from glassware (see [[Beckmann mixture]]). Chromic acid is also used for chrome plating baths, engraving etching, tanning, and as a colorant in glass and ceramics. | ||
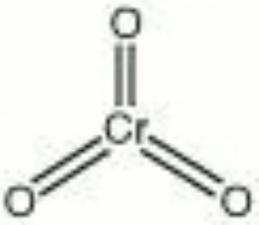
| − | + | [[[SliderGallery rightalign|chromium trioxide.jpg~Chemical structure]]] | |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
chromic acid; chromic anhydride; chromium (VI) oxide; anhydride chromique (Fr.); anidride cromica (It.); Chromsaeureanhydrid (Deut.); Chromtrioxid (Deut.); chroomtrioxyde (Ned.); | chromic acid; chromic anhydride; chromium (VI) oxide; anhydride chromique (Fr.); anidride cromica (It.); Chromsaeureanhydrid (Deut.); Chromtrioxid (Deut.); chroomtrioxyde (Ned.); | ||
| − | + | == Risks == | |
| − | == | + | * Powerful oxidizing agent, may explode on contact with reducing agents. |
| + | * Toxic by ingestion. | ||
| + | * Contact will corrode skin and membranes. | ||
| + | * Carcinogenic. | ||
| + | * Hygroscopic. | ||
| + | * Corrodes steel, copper and alloys. | ||
| + | * Decomposition produces toxic fumes. | ||
| + | * ThermoFisher: [https://www.fishersci.com/store/msds?partNumber=AC196610250&productDescription=CHROMIUM%28VI%29+OXIDE%2C+99%2B%25+25GR&vendorId=VN00032119&countryCode=US&language=en SDS] | ||
| + | ==Physical and Chemical Properties== | ||
| + | |||
Soluble in water, ethanol, mineral acids. | Soluble in water, ethanol, mineral acids. | ||
| Line 22: | Line 31: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 197 | + | | 197 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 2.70 | + | | 2.70 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 31: | Line 40: | ||
|- | |- | ||
! scope="row"| Boiling Point | ! scope="row"| Boiling Point | ||
| − | | 250 (dec) | + | | 250 C (dec) |
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 192 | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 192 | ||
| Line 50: | Line 51: | ||
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: Entry 2283 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: Entry 2283 | ||
| − | * | + | * Conservation termlist: www.hants.org.uk/museums |
| − | * Wikipedia | + | * Wikipedia: http://en.wikipedia.org/wiki/Chromium_trioxide (Accessed Jan. 15, 2006) |
* Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, ''Technology and Conservation'', Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985 | * Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, ''Technology and Conservation'', Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985 | ||
Latest revision as of 14:31, 29 May 2022
Description
Dark, violet-red deliquescent crystals. Chromium trioxide is normally used as an aqueous solution which is commonly called Chromic acid. Chromic acid is made by mixing Potassium dichromate and an acid such as Hydrochloric acid or Sulfuric acid. It is an extremely strong oxidizing agent that will react violently with any organic compound. For many years, chromic acid was used in chemical laboratories cleaning baths to remove all traces of organic residues from glassware (see Beckmann mixture). Chromic acid is also used for chrome plating baths, engraving etching, tanning, and as a colorant in glass and ceramics.
Synonyms and Related Terms
chromic acid; chromic anhydride; chromium (VI) oxide; anhydride chromique (Fr.); anidride cromica (It.); Chromsaeureanhydrid (Deut.); Chromtrioxid (Deut.); chroomtrioxyde (Ned.);
Risks
- Powerful oxidizing agent, may explode on contact with reducing agents.
- Toxic by ingestion.
- Contact will corrode skin and membranes.
- Carcinogenic.
- Hygroscopic.
- Corrodes steel, copper and alloys.
- Decomposition produces toxic fumes.
- ThermoFisher: SDS
Physical and Chemical Properties
Soluble in water, ethanol, mineral acids.
| Composition | CrO3 |
|---|---|
| CAS | 1333-82-0 |
| Melting Point | 197 C |
| Density | 2.70 g/ml |
| Molecular Weight | mol. wt. = 100.01 |
| Boiling Point | 250 C (dec) |
Resources and Citations
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 192
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: Entry 2283
- Conservation termlist: www.hants.org.uk/museums
- Wikipedia: http://en.wikipedia.org/wiki/Chromium_trioxide (Accessed Jan. 15, 2006)
- Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979