Difference between revisions of "Dammar"
| (6 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
| − | [[File:66-84_Dewaxed.Dammar_canvas.jpg|thumb|Dammar]] | + | [[File:66-84_Dewaxed.Dammar_canvas.jpg|thumb|Dammar on canvas (visible light left; UV light right)]] |
== Description == | == Description == | ||
A clear, pale yellow natural resin derived from ''Dipterocarpaceae'' trees growing in southeast Asia. The principal trees that supply dammar resin are of the genera ''Shorea, Balanocarpus,'' or ''Hopea''. Dammar is a triterpenoid resin that primarily contains dammarolic acid (C54H77O3(COOH)2). The soft, viscous, highly aromatic resin oozes readily from incisions in the bark and dries to become transparent, brittle, odorless lumps that are sorted into three grades: A (superior), B (small amount of impurities), and C (many impurities). To prepared as a varnish, dammar pieces are placed in a [[cheesecloth]] bag partially submersed in [[turpentine (oil)|turpentine]]. After a few hours, the dammar is dissolved and any residual material remaining in the bag is thrown out. This forms a high-quality, clear varnish for paintings. However, the presence of water during application may cause it to dry with a whitish bloom. Dammar was first introduced as a picture varnish in 1826 and by the end of the 19th century, its use surpassed [[mastic resin|mastic]]. It is also used in [[printing ink|printing inks]], [[lacquer, synthetic|cellulosic lacquers]], [[baked enamel|alkyd baking enamels]], and coatings for papers and textiles. Dammar solutions in [[chloroform]] or [[xylenes]] have been used for mounting thin sections for microscopic examination. | A clear, pale yellow natural resin derived from ''Dipterocarpaceae'' trees growing in southeast Asia. The principal trees that supply dammar resin are of the genera ''Shorea, Balanocarpus,'' or ''Hopea''. Dammar is a triterpenoid resin that primarily contains dammarolic acid (C54H77O3(COOH)2). The soft, viscous, highly aromatic resin oozes readily from incisions in the bark and dries to become transparent, brittle, odorless lumps that are sorted into three grades: A (superior), B (small amount of impurities), and C (many impurities). To prepared as a varnish, dammar pieces are placed in a [[cheesecloth]] bag partially submersed in [[turpentine (oil)|turpentine]]. After a few hours, the dammar is dissolved and any residual material remaining in the bag is thrown out. This forms a high-quality, clear varnish for paintings. However, the presence of water during application may cause it to dry with a whitish bloom. Dammar was first introduced as a picture varnish in 1826 and by the end of the 19th century, its use surpassed [[mastic resin|mastic]]. It is also used in [[printing ink|printing inks]], [[lacquer, synthetic|cellulosic lacquers]], [[baked enamel|alkyd baking enamels]], and coatings for papers and textiles. Dammar solutions in [[chloroform]] or [[xylenes]] have been used for mounting thin sections for microscopic examination. | ||
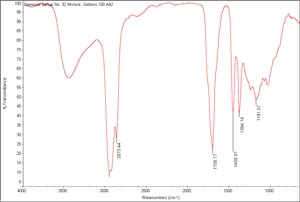
| − | + | [[[SliderGallery rightalign|Dewaxed Damar No. 32 Mixture, Gettens 100.A42.TIF~FTIR(MFA)]]] | |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
dammar (AAT, Esp., Fr., It.); damar (JAIC style guide); dammer; dammar gum; Malay dammar; Mata Kuching; cat's eye; Penak; gum Batu; Kalulut; Siput; Daging; Saraya; Kepong; Bata gum; Singapore dammar; Batavia dammar; Padang; Dipterocarpaceae | dammar (AAT, Esp., Fr., It.); damar (JAIC style guide); dammer; dammar gum; Malay dammar; Mata Kuching; cat's eye; Penak; gum Batu; Kalulut; Siput; Daging; Saraya; Kepong; Bata gum; Singapore dammar; Batavia dammar; Padang; Dipterocarpaceae | ||
| − | + | == Risks == | |
| − | == | + | * Low toxicity rating although dust inhalation may cause allergies. |
| + | * Darkens with age. | ||
| + | * Turns cloudy when moisture is present during preparation. | ||
| + | ==Physical and Chemical Properties== | ||
| − | Soluble in turpentine, oil, chloroform and aromatic hydrocarbons. | + | * Soluble in turpentine, oil, chloroform and aromatic hydrocarbons. |
| − | + | * Slightly soluble in ethanol. Insoluble in water or mineral spirits. | |
| − | Slightly soluble in ethanol. Insoluble in water or mineral spirits. | + | * Acid number = 16 -18; |
| − | + | * Saponification number = 20-65. | |
| − | Acid number = 16 -18; Saponification number = 20-65. | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 24: | Line 26: | ||
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 1.04-1.12 | + | | 1.04-1.12 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 32: | Line 34: | ||
| 1.515 -1.539 | | 1.515 -1.539 | ||
|} | |} | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
== Comparisons == | == Comparisons == | ||
| − | [[media: | + | [[media:download_file_105.pdf|Properties of Natural Resins]] |
| − | |||
| − | |||
| − | |||
| + | ==Resources and Citations== | ||
| + | * J.S. Mills, R.White, ''The Organic Chemistry of Museum Objects'', Butterworth Heinemann, London, 1994. | ||
| − | + | * L.Merz-Le, "Natural Resin Varnishes: Damar" AIC Painting Conservation Catalog, Varnishes and Surface Coatings, p.63, 1998. | |
* R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 Comment: dammar; melting point = 100-150C; specific gravity = 1.062 | * R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 Comment: dammar; melting point = 100-150C; specific gravity = 1.062 | ||
| − | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: Dammar. Retrieved May 26, 2003 | + | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: Dammar. Retrieved May 26, 2003. |
| − | * Wikipedia | + | * Wikipedia: http://en.wikipedia.org/wiki/Dammar (Accessed Jan. 25, 2006) melts at 120C |
* G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: dammar | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: dammar | ||
| Line 87: | Line 78: | ||
* Paintings Specialty Group, ''Painting Conservation Catalog'', Wendy Samet (ed.), AIC, Washington, DC, 1998 | * Paintings Specialty Group, ''Painting Conservation Catalog'', Wendy Samet (ed.), AIC, Washington, DC, 1998 | ||
| − | * Art and Architecture Thesaurus Online, | + | * Art and Architecture Thesaurus Online, https://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 Comment: dammar (preferred) |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 15:23, 14 July 2022
Description
A clear, pale yellow natural resin derived from Dipterocarpaceae trees growing in southeast Asia. The principal trees that supply dammar resin are of the genera Shorea, Balanocarpus, or Hopea. Dammar is a triterpenoid resin that primarily contains dammarolic acid (C54H77O3(COOH)2). The soft, viscous, highly aromatic resin oozes readily from incisions in the bark and dries to become transparent, brittle, odorless lumps that are sorted into three grades: A (superior), B (small amount of impurities), and C (many impurities). To prepared as a varnish, dammar pieces are placed in a Cheesecloth bag partially submersed in turpentine. After a few hours, the dammar is dissolved and any residual material remaining in the bag is thrown out. This forms a high-quality, clear varnish for paintings. However, the presence of water during application may cause it to dry with a whitish bloom. Dammar was first introduced as a picture varnish in 1826 and by the end of the 19th century, its use surpassed mastic. It is also used in printing inks, cellulosic lacquers, alkyd baking enamels, and coatings for papers and textiles. Dammar solutions in Chloroform or Xylenes have been used for mounting thin sections for microscopic examination.
Synonyms and Related Terms
dammar (AAT, Esp., Fr., It.); damar (JAIC style guide); dammer; dammar gum; Malay dammar; Mata Kuching; cat's eye; Penak; gum Batu; Kalulut; Siput; Daging; Saraya; Kepong; Bata gum; Singapore dammar; Batavia dammar; Padang; Dipterocarpaceae
Risks
- Low toxicity rating although dust inhalation may cause allergies.
- Darkens with age.
- Turns cloudy when moisture is present during preparation.
Physical and Chemical Properties
- Soluble in turpentine, oil, chloroform and aromatic hydrocarbons.
- Slightly soluble in ethanol. Insoluble in water or mineral spirits.
- Acid number = 16 -18;
- Saponification number = 20-65.
| Melting Point | 90 (softens); 100-180 (melts) |
|---|---|
| Density | 1.04-1.12 g/ml |
| Molecular Weight | Tg =39.3 C |
| Refractive Index | 1.515 -1.539 |
Comparisons
Resources and Citations
- J.S. Mills, R.White, The Organic Chemistry of Museum Objects, Butterworth Heinemann, London, 1994.
- L.Merz-Le, "Natural Resin Varnishes: Damar" AIC Painting Conservation Catalog, Varnishes and Surface Coatings, p.63, 1998.
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966 Comment: dammar; melting point = 100-150C; specific gravity = 1.062
- Encyclopedia Britannica, http://www.britannica.com Comment: Dammar. Retrieved May 26, 2003.
- Wikipedia: http://en.wikipedia.org/wiki/Dammar (Accessed Jan. 25, 2006) melts at 120C
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: dammar
- Reed Kay, The Painter's Guide To Studio Methods and Materials, Prentice-Hall, Inc., Englewood Cliffs, NJ, 1983
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing) Comment: damar
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993 Comment: dammar
- Hermann Kuhn, Conservation and Restoration of Works of Art and Antiquities, Butterworths, London, 1986 Comment: softens at 90C, melting point at 180C
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979 Comment: damar
- C.V.Horie, Materials for Conservation, Butterworth-Heineman, London, 1997
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982 Comment: dammar
- Polymer Handbook, Sealants and Adhesives
- John S. Mills, Raymond White, The Organic Chemistry of Museum Objects, Butterworth Heineman, London, 2nd ed., 1994
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry #2872 - damar
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998 Comment: dammar
- Thomas B. Brill, Light Its Interaction with Art and Antiquities, Plenum Press, New York City, 1980
- Paintings Specialty Group, Painting Conservation Catalog, Wendy Samet (ed.), AIC, Washington, DC, 1998
- Art and Architecture Thesaurus Online, https://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 Comment: dammar (preferred)