Difference between revisions of "Diacetone alcohol"
Jump to navigation
Jump to search
| (3 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | A strong solvent with a minty odor that can soften dried [[linseed oil]] films (Mayer 1969). Diacetone alcohol is used as a solvent for [[cellulose nitrate]], [[cellulose acetate]], [[oil|oils]], [[natural resin|resin]], [[wax|waxes]], [[fat|fats]], [[tar|tars]], [[lacquer, synthetic|lacquers]], [[ | + | A strong solvent with a minty odor that can soften dried [[linseed oil]] films (Mayer 1969). Diacetone alcohol is used as a solvent for [[cellulose nitrate]], [[cellulose acetate]], [[oil|oils]], [[natural resin|resin]], [[wax|waxes]], [[fat|fats]], [[tar|tars]], [[lacquer, synthetic|lacquers]], [[dye|dyes]], and [[oil stain|oil stains]]. It is also used for preserving wood, leather, and animal tissue and for cleaning metals and textiles. Toch (1931) mentions diacetone alcohol for coalescing crackle on old varnish films. |
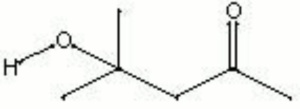
| − | + | [[[SliderGallery rightalign|diacetone alcohol.jpg~Chemical structure]]] | |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
diacetone; 4-hydroxy-4-methylpentanone-2; pyranton | diacetone; 4-hydroxy-4-methylpentanone-2; pyranton | ||
| − | + | == Risks == | |
| − | == | + | * Flammable, fire risk. |
| + | * Overexposure may cause irritation of the respiratory system. | ||
| + | * Fisher Scientific: [https://fscimage.fishersci.com/msds/06460.htm MSDS] | ||
| + | == Physical and Chemical Properties == | ||
Miscible with alcohols, aromatics, halogenated hydrocarbons, esters and water. | Miscible with alcohols, aromatics, halogenated hydrocarbons, esters and water. | ||
| Line 22: | Line 25: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | -44 | + | | -44 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 0.9306 | + | | 0.9306 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 34: | Line 37: | ||
|- | |- | ||
! scope="row"| Boiling Point | ! scope="row"| Boiling Point | ||
| − | | 167.9 | + | | 167.9 C |
|} | |} | ||
| − | == | + | == Resources and Citations == |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | * M. Toch, ''Paint, Paintings and Restoration'', D.Van Nostrand, New York, 1931. | |
* R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 | * R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 | ||
Latest revision as of 13:00, 19 July 2022
Description
A strong solvent with a minty odor that can soften dried Linseed oil films (Mayer 1969). Diacetone alcohol is used as a solvent for Cellulose nitrate, Cellulose acetate, oils, resin, waxes, fats, tars, lacquers, dyes, and oil stains. It is also used for preserving wood, leather, and animal tissue and for cleaning metals and textiles. Toch (1931) mentions diacetone alcohol for coalescing crackle on old varnish films.
Synonyms and Related Terms
diacetone; 4-hydroxy-4-methylpentanone-2; pyranton
Risks
- Flammable, fire risk.
- Overexposure may cause irritation of the respiratory system.
- Fisher Scientific: MSDS
Physical and Chemical Properties
Miscible with alcohols, aromatics, halogenated hydrocarbons, esters and water.
| Composition | CH3COCH2C(CH3)2OH |
|---|---|
| CAS | 123-42-2 |
| Melting Point | -44 C |
| Density | 0.9306 g/ml |
| Molecular Weight | mol. wt. = 116.16 |
| Refractive Index | 1.4232 |
| Boiling Point | 167.9 C |
Resources and Citations
- M. Toch, Paint, Paintings and Restoration, D.Van Nostrand, New York, 1931.
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 9
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 3008