Difference between revisions of "Magnesite, natural"
| (6 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
| + | [[File:20.231-CR9283-d1.jpg|thumb|Nubian shawabty<br>MFA# 20.231]] | ||
[[File:pm30415magnesite.jpg|thumb|Magnesite]] | [[File:pm30415magnesite.jpg|thumb|Magnesite]] | ||
== Description == | == Description == | ||
| − | A naturally occurring mineral composed of [[magnesium%20carbonate|magnesium carbonate]] that is the primary commercial source of [[magnesium|magnesium]]. Magnesite deposits have been mined in Russia, Austria (Radenthein), Greece, Norway, India, China (Liaotung); Australia, Canada (Quebec) and the United States (Nevada, New Mexico, California, Alaska). The hexagonal mineral usually occurs in large masses. Magnesite can be transparent to opaque with colors of white, yellow, gray or pale brown due to the presence of impurities. Most magnesite is formed from the action of carbonated water on magnesium-rich ores. Powdered magnesite is used as a filler and inert pigment. Blocks of magnesite are used as a refractory material for furnace linings. Calcined magnesite is used in [[oxychloride%20cement|oxychloride cement]]. | + | A naturally occurring mineral composed of [[magnesium%20carbonate|magnesium carbonate]] that is the primary commercial source of [[magnesium|magnesium]]. Magnesite deposits have been mined in Russia, Austria (Radenthein), Greece, Norway, India, China (Liaotung); Australia, Canada (Quebec) and the United States (Nevada, New Mexico, California, Alaska). The hexagonal mineral usually occurs in large masses. Magnesite can be transparent to opaque with colors of white, yellow, gray or pale brown due to the presence of impurities. It has also been dyed to imitate other stones. Most magnesite is formed from the action of carbonated water on magnesium-rich ores. Powdered magnesite is used as a filler and inert pigment. Blocks of magnesite are used as a refractory material for furnace linings. Calcined magnesite is used in [[oxychloride%20cement|oxychloride cement]]. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
magnesia (incorrect); magnesia alba; magnesia white; Pigment White 18; magnesita (Esp.); magnésite (Fr.); Magnesit (Deut.); magnesiet (Ned.) | magnesia (incorrect); magnesia alba; magnesia white; Pigment White 18; magnesita (Esp.); magnésite (Fr.); Magnesit (Deut.); magnesiet (Ned.) | ||
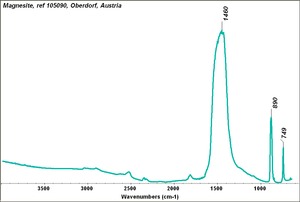
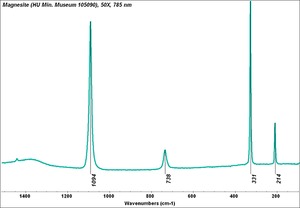
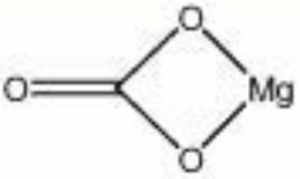
| + | [[[SliderGallery rightalign|Magnesite.TIF~FTIR (MFA)|Magnesite (HU Min. Museum 105090), 50X, 785 nm copy.tif~Raman (MFA)|magnesite, natural.jpg~Chemical structure]]] | ||
| + | == Risks == | ||
| + | * Nontoxic. | ||
| + | * Ingestion has a laxative effect. | ||
| + | * Noncombustible. | ||
| + | * ThermoFisher: [https://www.fishersci.com/store/msds?partNumber=M263&productDescription=MAGNESIUM+CARBONATE+PURIF+3KG&vendorId=VN00033897&countryCode=US&language=en SDS] | ||
| + | == Physical and Chemical Properties == | ||
| − | + | * Hexagonal system with rhombohedral cleavage that is perfect in three directions. | |
| − | + | * Fracture = conchoidal to granular | |
| − | + | * Luster = vitreous to silky | |
| − | + | * Streak = white | |
| − | Hexagonal system with rhombohedral cleavage that is perfect in three directions. | + | * Fluorescence = generally inert; may exhibit pale blue or green fluorescence and phosphorescence in UV |
| − | + | * Microscopically, particles appear as translucent, colorless, angular crystals. They exhibit high birefringence under crossed polars. Extinction is complete and straight. | |
| − | Fracture = conchoidal | ||
| − | |||
| − | Microscopically, particles appear as translucent, colorless, angular crystals. They exhibit high | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 22: | Line 27: | ||
! scope="row"| Composition | ! scope="row"| Composition | ||
| MgCO3 | | MgCO3 | ||
| − | |||
| − | |||
| − | |||
|- | |- | ||
! scope="row"| Mohs Hardness | ! scope="row"| Mohs Hardness | ||
| Line 30: | Line 32: | ||
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 3.0-3. | + | | 3.0-3.2 g/ml |
| − | |||
| − | |||
| − | |||
|- | |- | ||
! scope="row"| Refractive Index | ! scope="row"| Refractive Index | ||
| 1.508; 1.510; 1.700 | | 1.508; 1.510; 1.700 | ||
| + | |- | ||
| + | ! scope="row"| Birefringence | ||
| + | | 0.191 | ||
|} | |} | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
== Comparisons == | == Comparisons == | ||
| Line 53: | Line 45: | ||
[[media:download_file_534.pdf|Characteristics of Common White Pigments]] | [[media:download_file_534.pdf|Characteristics of Common White Pigments]] | ||
| − | + | ==Resources and Citations== | |
| − | + | * Mineralogy Database: [http://www.webmineral.com/data/Magnesite.shtml Magnesite] | |
| − | == | + | * Gem Identification Lab Manual, Gemological Institute of America, 2016. |
| − | |||
* Helmut Schweppe, Schweppe color collection index and information book | * Helmut Schweppe, Schweppe color collection index and information book | ||
| − | |||
* Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, ''Pigment Compendium'', Elsevier Butterworth-Heinemann, Oxford, 2004 | * Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, ''Pigment Compendium'', Elsevier Butterworth-Heinemann, Oxford, 2004 | ||
| − | |||
* Ralph Mayer, ''A Dictionary of Art Terms and Techniques'', Harper and Row Publishers, New York, 1969 (also 1945 printing) | * Ralph Mayer, ''A Dictionary of Art Terms and Techniques'', Harper and Row Publishers, New York, 1969 (also 1945 printing) | ||
| − | |||
* A.Lucas, J.R.Harris, ''Ancient Egyptian Materials and Industries'', Edward Arnold Publishers Ltd., London, 4th edition, 1962 | * A.Lucas, J.R.Harris, ''Ancient Egyptian Materials and Industries'', Edward Arnold Publishers Ltd., London, 4th edition, 1962 | ||
| − | + | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: "magnesite" [Accessed December 4, 2001]. | |
| − | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: "magnesite" | ||
| − | |||
* C.W.Chesterman, K.E.Lowe, ''Audubon Society Field Guide to North American Rocks and Minerals'', Alfred A. Knopf, New York, 1979 | * C.W.Chesterman, K.E.Lowe, ''Audubon Society Field Guide to North American Rocks and Minerals'', Alfred A. Knopf, New York, 1979 | ||
| − | + | * Wikipedia: [https://en.wikipedia.org/wiki/Magnesite Magnesite] (Accessed Sept. 7, 2005 and Dec 2022) | |
| − | * Wikipedia | ||
| − | |||
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
| − | |||
* ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | * ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | ||
| − | |||
* ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | * ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | ||
Latest revision as of 13:26, 24 January 2023
Description
A naturally occurring mineral composed of Magnesium carbonate that is the primary commercial source of Magnesium. Magnesite deposits have been mined in Russia, Austria (Radenthein), Greece, Norway, India, China (Liaotung); Australia, Canada (Quebec) and the United States (Nevada, New Mexico, California, Alaska). The hexagonal mineral usually occurs in large masses. Magnesite can be transparent to opaque with colors of white, yellow, gray or pale brown due to the presence of impurities. It has also been dyed to imitate other stones. Most magnesite is formed from the action of carbonated water on magnesium-rich ores. Powdered magnesite is used as a filler and inert pigment. Blocks of magnesite are used as a refractory material for furnace linings. Calcined magnesite is used in Oxychloride cement.
Synonyms and Related Terms
magnesia (incorrect); magnesia alba; magnesia white; Pigment White 18; magnesita (Esp.); magnésite (Fr.); Magnesit (Deut.); magnesiet (Ned.)
Risks
- Nontoxic.
- Ingestion has a laxative effect.
- Noncombustible.
- ThermoFisher: SDS
Physical and Chemical Properties
- Hexagonal system with rhombohedral cleavage that is perfect in three directions.
- Fracture = conchoidal to granular
- Luster = vitreous to silky
- Streak = white
- Fluorescence = generally inert; may exhibit pale blue or green fluorescence and phosphorescence in UV
- Microscopically, particles appear as translucent, colorless, angular crystals. They exhibit high birefringence under crossed polars. Extinction is complete and straight.
| Composition | MgCO3 |
|---|---|
| Mohs Hardness | 3.5 - 4.5 |
| Density | 3.0-3.2 g/ml |
| Refractive Index | 1.508; 1.510; 1.700 |
| Birefringence | 0.191 |
Comparisons
Characteristics of Common White Pigments
Resources and Citations
- Mineralogy Database: Magnesite
- Gem Identification Lab Manual, Gemological Institute of America, 2016.
- Helmut Schweppe, Schweppe color collection index and information book
- Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, Pigment Compendium, Elsevier Butterworth-Heinemann, Oxford, 2004
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- A.Lucas, J.R.Harris, Ancient Egyptian Materials and Industries, Edward Arnold Publishers Ltd., London, 4th edition, 1962
- Encyclopedia Britannica, http://www.britannica.com Comment: "magnesite" [Accessed December 4, 2001].
- C.W.Chesterman, K.E.Lowe, Audubon Society Field Guide to North American Rocks and Minerals, Alfred A. Knopf, New York, 1979
- Wikipedia: Magnesite (Accessed Sept. 7, 2005 and Dec 2022)
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998