Difference between revisions of "Potassium ferricyanide"
Jump to navigation
Jump to search
| (3 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | Ruby red crystalline powder that is used to make blueprints, to stain [[wood]], and dye [[wool]]. Potassium ferricyanide is also used as an etching liquid in [[electroplate|electroplating]] and as a [[reducing agent]] for photography. | + | Ruby red crystalline powder that is used to make [[blueprint paper|blueprints]], to stain [[wood]], and dye [[wool]]. Potassium ferricyanide is also used as an etching liquid in [[electroplate|electroplating]] and as a [[reducing agent]] for photography. |
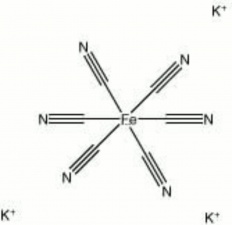
| − | + | [[[SliderGallery rightalign|potassium ferricyanide.jpg~Chemical structure]]] | |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
farmer's reducer; potassium hexacyanoferrate (III); Mercer's liquor; red prussiate of potash; red potassium prussiate; | farmer's reducer; potassium hexacyanoferrate (III); Mercer's liquor; red prussiate of potash; red potassium prussiate; | ||
| − | [ | + | == Risks == |
| + | |||
| + | * Decomposes with heat, acids or UV light to produce highly toxic hydrogen cyanide fumes. | ||
| + | * ThermoFisher: [https://www.fishersci.com/store/msds?partNumber=P232500&productDescription=POT+FERRICYANIDE+CERT+ACS+500G&vendorId=VN00033897&countryCode=US&language=en SDS] | ||
| − | == | + | ==Physical and Chemical Properties== |
Soluble in water. Slightly soluble in ethanol. | Soluble in water. Slightly soluble in ethanol. | ||
| Line 22: | Line 25: | ||
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 1.85 | + | | 1.85 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 28: | Line 31: | ||
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 632 | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 632 | ||
| Line 46: | Line 39: | ||
* Michael McCann, ''Artist Beware'', Watson-Guptill Publications, New York City, 1979 | * Michael McCann, ''Artist Beware'', Watson-Guptill Publications, New York City, 1979 | ||
| − | * | + | * Photographic chemicals at www.jetcity.com/~mrjones/chemdesc |
| − | * N.Odegaard, S.Carroll, W.Zimmt, ''Material Characterization Tests for Objects of Art and Archaeology'', Archetype Publications, London, 2000 | + | * N.Odegaard, S.Carroll, W.Zimmt, ''Material Characterization Tests for Objects of Art and Archaeology'', Archetype Publications, London, 2000, p. 62. |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 12:28, 8 September 2022
Description
Ruby red crystalline powder that is used to make blueprints, to stain Wood, and dye Wool. Potassium ferricyanide is also used as an etching liquid in electroplating and as a Reducing agent for photography.
Synonyms and Related Terms
farmer's reducer; potassium hexacyanoferrate (III); Mercer's liquor; red prussiate of potash; red potassium prussiate;
Risks
- Decomposes with heat, acids or UV light to produce highly toxic hydrogen cyanide fumes.
- ThermoFisher: SDS
Physical and Chemical Properties
Soluble in water. Slightly soluble in ethanol.
| Composition | K3Fe(CN)6 |
|---|---|
| CAS | 13746-66-2 |
| Density | 1.85 g/ml |
| Molecular Weight | mol. wt. = 329.25 |
Resources and Citations
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 632
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Photographic chemicals at www.jetcity.com/~mrjones/chemdesc
- N.Odegaard, S.Carroll, W.Zimmt, Material Characterization Tests for Objects of Art and Archaeology, Archetype Publications, London, 2000, p. 62.