Difference between revisions of "Cobalt violet, deep"
Jump to navigation
Jump to search
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
|||
| (2 intermediate revisions by one other user not shown) | |||
| Line 8: | Line 8: | ||
cobalt phosphate; deep cobalt violet; Pigment Violet 14; CI 77360; violeta de cobalto intenso (Esp.); violet de cobalt (foncé) (Fr.); Kobaltviolett (dunkel) (Deut.); iodes toy kobaltioy skoyro (Gr.); violetto di cobalto scuro (It.); cobalt violet (donker) (Ned.); violeta de cobalto, escuro (Port.) | cobalt phosphate; deep cobalt violet; Pigment Violet 14; CI 77360; violeta de cobalto intenso (Esp.); violet de cobalt (foncé) (Fr.); Kobaltviolett (dunkel) (Deut.); iodes toy kobaltioy skoyro (Gr.); violetto di cobalto scuro (It.); cobalt violet (donker) (Ned.); violeta de cobalto, escuro (Port.) | ||
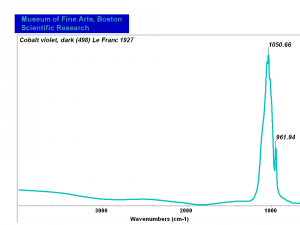
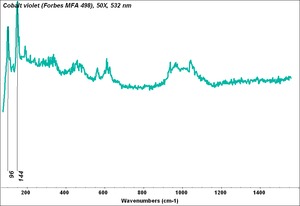
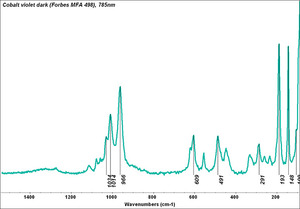
| − | [[[SliderGallery rightalign|MFA | + | [[[SliderGallery rightalign|Cobalt Violet, dark (498).PNG ~FTIR (MFA) (Forbes 498)|Cobalt violet (Forbes MFA 498), 50X, 532 nm.TIF~Raman (MFA) (532nm)|Cobalt violet dark (Forbes MFA 498), 785nm resize.tif~Raman (MFA) (785nm)|f498sem.jpg~SEM (MFA)|f498edsbw.jpg~EDS (MFA)]]] |
| + | == Risks == | ||
| − | + | * Skin contact may cause allergies, especially on elbows, neck and ankles. | |
| + | * Chronic inhalation may cause asthma. | ||
| + | * Ingestion may cause vomiting, diarrhea and the sensation of hotness | ||
| − | Microscopically, the particles are strongly pleochroic with colors from pink to violet to yellow. Highly birefringent under crossed polars. Appears magenta with Chelsea filter. | + | ==Physical and Chemical Properties== |
| + | |||
| + | * Microscopically, the particles are strongly pleochroic with colors from pink to violet to yellow. | ||
| + | * Highly birefringent under crossed polars. | ||
| + | * Appears magenta with Chelsea filter. | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 23: | Line 30: | ||
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | * Pigments Through the Ages: [http://webexhibits.org/pigments/indiv/overview/coviolet.html Cobalt violet] | |
| − | + | * Corbeil, Marie-Claude, Jean-Pierre Charland, Elizabeth Moffatt. 'The characterization of cobalt violet pigments' ''Studies in Conservation'' vol.47 (2002), pp.237-249. | |
* Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, ''Pigment Compendium'', Elsevier Butterworth-Heinemann, Oxford, 2004 | * Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, ''Pigment Compendium'', Elsevier Butterworth-Heinemann, Oxford, 2004 | ||
Latest revision as of 12:10, 30 May 2022
Description
A medium to strong violet pigment composed of Cobaltous phosphate. Deep cobalt violet was first developed in 1859 by Salvetat and sold as an artists' pigment in 1890. The colorfast pigment has low tinting strength and dries quickly in oil paints.
Synonyms and Related Terms
cobalt phosphate; deep cobalt violet; Pigment Violet 14; CI 77360; violeta de cobalto intenso (Esp.); violet de cobalt (foncé) (Fr.); Kobaltviolett (dunkel) (Deut.); iodes toy kobaltioy skoyro (Gr.); violetto di cobalto scuro (It.); cobalt violet (donker) (Ned.); violeta de cobalto, escuro (Port.)
Risks
- Skin contact may cause allergies, especially on elbows, neck and ankles.
- Chronic inhalation may cause asthma.
- Ingestion may cause vomiting, diarrhea and the sensation of hotness
Physical and Chemical Properties
- Microscopically, the particles are strongly pleochroic with colors from pink to violet to yellow.
- Highly birefringent under crossed polars.
- Appears magenta with Chelsea filter.
| Composition | Co3(PO4)2 - 8H2O |
|---|---|
| Refractive Index | >1.662 |
Resources and Citations
- Pigments Through the Ages: Cobalt violet
- Corbeil, Marie-Claude, Jean-Pierre Charland, Elizabeth Moffatt. 'The characterization of cobalt violet pigments' Studies in Conservation vol.47 (2002), pp.237-249.
- Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, Pigment Compendium, Elsevier Butterworth-Heinemann, Oxford, 2004
- Reed Kay, The Painter's Guide To Studio Methods and Materials, Prentice-Hall, Inc., Englewood Cliffs, NJ, 1983
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000
- Thomas B. Brill, Light Its Interaction with Art and Antiquities, Plenum Press, New York City, 1980