Difference between revisions of "Polyisoprene"
Jump to navigation
Jump to search
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
|||
| (4 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | A thermoplastic, rubbery polymer. Polyisoprene (cis-1,4-polyisoprene), is the primary component in [ | + | A thermoplastic, rubbery polymer. Polyisoprene (cis-1,4-polyisoprene), is the primary component in [[caoutchouc]] that was used to make [[rubber (natural, vulcanized)|vulcanized natural rubber]]. Both the cis and trans forms of the polymer may be produced synthetically. The trans form resembles [[gutta-percha|gutta-percha]]. Both types can be vulcanized with [[sulfur|sulfur]]. |
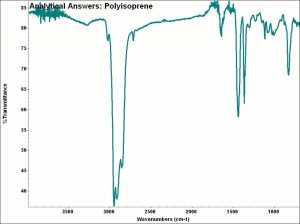
| − | + | [[[SliderGallery rightalign|aaiP-ISOPRN.jpg~FTIR]]] | |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
polyisoprène (Fr.); poliisopreno (Esp.); polisoprene (It.); poliisopreno (Port.); cis-1,4-polyisoprene; trans-1,4-polyisoprene; | polyisoprène (Fr.); poliisopreno (Esp.); polisoprene (It.); poliisopreno (Port.); cis-1,4-polyisoprene; trans-1,4-polyisoprene; | ||
| − | + | == Physical and Chemical Properties == | |
| − | |||
| − | == | ||
| − | Soluble in aliphatic and aromatic solvents. Insoluble in acetone, diethyl ether. Burns with a dark yellow, sooty flame that smells like burnt rubber. | + | * Soluble in aliphatic and aromatic solvents. Insoluble in acetone, diethyl ether. |
| + | * Burns with a dark yellow, sooty flame that smells like burnt rubber. | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 28: | Line 27: | ||
[[media:download_file_315.pdf|Physical Properties for Selected Thermoset Resins]] | [[media:download_file_315.pdf|Physical Properties for Selected Thermoset Resins]] | ||
| − | + | == Resources and Citations == | |
| − | |||
| − | == | ||
* G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 676 | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 676 | ||
| Line 36: | Line 33: | ||
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
| − | * Art and Architecture Thesaurus Online, | + | * Art and Architecture Thesaurus Online, https://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 12:33, 22 October 2022
Description
A thermoplastic, rubbery polymer. Polyisoprene (cis-1,4-polyisoprene), is the primary component in Caoutchouc that was used to make vulcanized natural rubber. Both the cis and trans forms of the polymer may be produced synthetically. The trans form resembles Gutta-percha. Both types can be vulcanized with Sulfur.
Synonyms and Related Terms
polyisoprène (Fr.); poliisopreno (Esp.); polisoprene (It.); poliisopreno (Port.); cis-1,4-polyisoprene; trans-1,4-polyisoprene;
Physical and Chemical Properties
- Soluble in aliphatic and aromatic solvents. Insoluble in acetone, diethyl ether.
- Burns with a dark yellow, sooty flame that smells like burnt rubber.
| Composition | (C5H8)n |
|---|---|
| CAS | 9003-31-0 |
Comparisons
General Characteristics of Polymers
Physical Properties for Selected Thermoset Resins
Resources and Citations
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 676
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Art and Architecture Thesaurus Online, https://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000